Introduction
Glioblastoma, a grade IV astrocytoma, is a fast-growing brain tumor arising de novo or evolving from astrocytes, the supportive cells in the brain.1,2 The World Health Organization defines grade IV tumors as mitotically active, necrotic, and malignant. Further, the isocitrate dehydrogenase wildtype grade IV glioblastoma is a mutational state marked by histological hypercellularity, microvascular proliferation, and necrosis.3,4
The incidence of glioblastoma is 2-3 cases in 100,000 of the population, which is the highest among malignant brain tumors, accounting for 60 percent of diagnosed cases.5 A recent single-institution retrospective study of 60 patients found that the overall survival rate was 30 percent at one year and 6.7 percent at two years after a maximum resection followed by chemoradiotherapy and adjuvant temozolomide chemotherapy.6 Other prognostic data calculated the median survival time of 14.6 months, a 26.5 percent two-year survival rate, and a five-year survival rate of less than 5 percent.7 The glioblastoma growth rate is 1.4 percent per day, and the volume doubling time occurs in 49.6 days, contributing to its dismal prognosis.8 With treatment, most patients experience tumor recurrence with a diminished prognosis.9
The cerebral hemispheres, specifically the supratentorial major lobes, are common sites for glioblastoma.1 Before treatment, brain tumors can alter the intracranial contents, causing unilateral headaches in 30-50 percent of cases, visual disturbances from focal neural deficits, and seizures.10 Tumor growth produces hallucinations from seizure activity.11 Hallucination types vary by etiology and pathological location.10 Visual field deficits occur from tissue destruction affecting the visual pathway—supramarginal gyrus, angular gyrus, and tumors in the parietal-occipital junction.12 Acute subjective complaints of visual disturbances in the form of complex hallucinations or visual field loss present an opportunity for early diagnosis, lesion localization, and swift intervention.
The parietal neurons contribute to sensorimotor function as an interface between perception and action.13 The spatial perception stimuli received by parietal lobe neurons originate from receptor surfaces such as the retina.14 The parietal cortex contains a complex neuronal construct subject to visuospatial perception dysfunction, particularly with right parietal lobe damage.15 Surgical removal of parietal lobe tumors is the mainstay of treatment, during which impairment of any sensorimotor functions is likely before and after the resection. The case study subject experienced postoperative sensory deficits that are well-documented in neuroscience, such as a lapse in determining the perceptual orientation of extrinsic objects in his extra personal space.16
Case Report
A 76-year-old man presented for an urgent evaluation with complaints of a recent onset of intermittent kaleidoscope-like transient disturbances in his vision that alternated between his right and left sides for ten-minute durations. He noticed multiple episodes a day for one month. He also described seeing images of people and cars moving past him on his left side. He recounted the actions of the people he saw in his hallucinations as bending down and wearing red tee shirts. The visual images appeared with opened or closed eyes. He had no blurred vision, transient vision loss, or diplopia. He had no history of head trauma or eye injury. He complained of headaches that he attributed to neck arthritis. The head pain originated in the lateral neck radiating bilaterally to the parietal area of his head. His headaches were constant with increasing severity for two months. He admitted waking with a headache. He denied nausea or vomiting, strange smells, limb paralysis, or cognitive changes.
His medical history included psychiatric care for anxiety disorder for eleven years, with no recent psychiatric visits and no prescribed antipsychotic medications. He admitted well-controlled hypertension and hypercholesterolemia. His systemic medications included the diuretic hydrochlorothiazide (Sandoz, Germany), atenolol (Sandoz, Germany) for hypertension, and simvastatin (Zocor, Merk & Co., Rahway, NJ) for hyperlipidemia. He had no history of cancer and no known medical or environmental allergies.
His best corrected visual acuity was 20/20 in each eye. His ocular motor exams were normal. His neurological exam revealed a cranial nerve II transmission abnormality manifesting as a visual field defect. No gross abnormalities were detected in either cranial nerve I or III-XII. A dilated ocular health exam was completed and did not reveal contributory findings for his hallucinations and headache symptoms.
Optical coherence tomography (Zeiss, Dublin, CA) imaging of the macula and optic nerve was unremarkable. The automated Humphrey Visual Field 24-2 SITA-Faster test (Zeiss, Dublin, CA) revealed an incongruous left homonymous hemianopia denser inferiorly, as shown in a single visit structure-function analysis display (Figure 1).
His diagnoses included a neurological visual field defect, the suspicion of intracranial pathology with a progressive headache, intermittent visual disturbances, and complex hallucinations. The patient was educated on the risk of intracranial pathology, specifically a brain tumor, and the indication for neuroimaging.
Three days after his optometry visit, the radiology report detailed the findings of a 2.0 x 2.0 x 1.7 cm peripherally enhancing intra-axial hemorrhagic lesion within the inferior right parietal lobe with moderate surrounding white matter vasogenic edema without midline shift (Figure 2). The magnetic resonance imaging axial view showed the peripherally enhancing mass in the inferior right parietal lobe precuneus, effacing the white matter between the calcarine sulcus and parietal-occipital sulcus (Figure 3). The structural characteristics showed calcific or hemorrhagic properties denoted by the non-enhancing central darkness. The sagittal orientation of the neuroanatomy showed the mass location and tissue alteration proximal to the occipital lobe (Figure 4).
Four days after his optometry visit, he underwent a right parietal craniotomy with isocitrate dehydrogenase intracranial neurosurgical resection of a well-circumscribed isocitrate dehydrogenase wildtype glioblastoma measuring 2.8 × 1.5 × 1.2 cm concomitantly with radiation treatment. In addition, his oncology medical treatment included oral temozolomide (Merck, Sharp, Dohme, Rahway, NJ) 140 mg two hours before his radiation treatment five days a week for 12 weeks.
Optometry Follow-up
He presented three months postoperatively. His last visit with his neurosurgeon was one day prior, and he finished the first course of radiation and oral chemotherapy. He reported headache resolution and cessation of the visual hallucinations immediately following his procedure. He had no blurred or decreased central vision. He reported noticing a blind spot in his left temporal field after the surgery.
He reported fatigue and episodes of forgetfulness that he described as difficulty visualizing the location of objects in space since the procedure, a common symptom of the somatosensory dysfunction associated with parietal lobe disease. For example, he required multiple attempts to locate a wall light switch while reaching to turn it on. This phenomenon illustrated an example of a parietal lobe spatial perceptual deficit and explained his difficulty with visually guided movement coordination. Fortunately, with effort, he conducted his routine activities adequately.
His best corrected visual acuity remained 20/20 in each eye. No changes were detected in his afferent visual field testing and ocular motor testing. A healing 6 cm long surgical craniotomy incision extending anteriorly to posteriorly on the right-side parietal region was observed on a shaved area of his scalp. His anterior segment ocular health was unchanged. The dilated fundus exam showed no new pathological signs.
Follow-up optical coherence tomography studies of the optic nerve and retinal nerve fiber layer were unchanged in each eye. The postoperative automated visual field test in the single visit structure-function analysis (Figure 5) showed a persistent incongruous left homonymous hemianopia denser inferiorly respecting the vertical midline.
He was diagnosed with an incongruous left homonymous hemianopia denser inferiorly, corresponding to a history of a right parietal lobe glioblastoma status post right craniotomy with maximum resection followed by chemoradiotherapy and adjuvant temozolomide chemotherapy. He was educated about the spatial perception symptoms he experienced due to the tumor’s location. He was encouraged to log his experiences to discuss with his neurologist as he discovered the extent of his brain damage. His neurology follow-up was in one month when the second course of radiation and chemotherapy commenced.
Discussion
Pathological Clinical Considerations
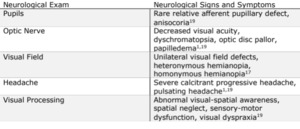
When glioblastoma induces an incongruous visual field homonymous hemianopia, the defect can be localized to the fibers of the geniculocalcarine tracts.17 Forming the dorsal optic radiation and Meyer’s loop through both parietal and temporal pathways, the geniculocalcarine tracts terminate in the visual cortex’s cuneus and lingual gyrus.16 As most clinical presentations of headaches are not subjected to neuroimaging, routine visual field testing is beneficial particularly when headache symptoms escalate to tumor suspicion. Headaches associated with glioblastoma can be severe, refractory, and accompanied by nausea and vomiting.18 Neurological symptoms often guide the eye exam and confirm the presence of a tumor (Table 1).19
The onset of visual disturbances can precede tumor diagnosis. Likewise, tumor induced seizures can trigger hallucinations, the perception of objects or visual events without an external stimulus.10 Three hallucination types include the psychophysiology pathogenesis of altered brain anatomy, the neurotransmitter-causing psychobiological, and psychodynamic hallucinations resulting from unconscious streams into consciousness.10 Complex hallucinations can result from disturbances of the interdependent relationship between brain anatomy and chemistry or seizure activity at visual processing cortical centers.10 Moreover, hallucinations involving vivid scenes, such as people carrying out tasks, are thought to be related to tumor activity.5
Magnetic resonance imaging is the most important diagnostic tool for glioblastoma detection.20 Glioblastomas showing substantial gadolinium contrast enhancement with central necrosis signal a higher-grade tumor.11 The magnetic resonance imaging spectroscopy modality is helpful in diagnosing challenging lesions.19 It provides a noninvasive analysis quantifying the chemical composition of metabolites in a tumor tissue sampling.19
Glioblastoma alterations such as the isocitrate dehydrogenase wildtype mutation are detected by immunohistochemistry, and notably, identifying intratumor genetic mutations can predict prognosis and treatment response.21 Immunohistochemistry enables pathologists to determine the exact type and subtype of cancers by looking for unique markers within cancer cells.22 It combines anatomical, immunological, and biochemical imaging techniques to visualize and document intracellular components using antibodies to bind to targeted antigens in situ.21
The aggressive nature of glioblastomas makes it impossible for neurosurgeons to cure the tumor entirely.1 The World Health Organization established a tumor proliferative index that increases with tumor grade.3 Thus, grade IV glioblastoma aggressively invades surrounding tissues.3 Glioblastoma treatment involves maximum tumor resection, radiotherapy, and temozolomide.5 Surgery is limited by the protocol to preserve adjacent brain tissue required for normal neurological function.3 In glioblastoma oncology treatment, temozolomide is an alkylating prodrug used to cross the blood-brain barrier and disrupt cell DNA structure by delivering a methyl group to purine bases of DNA (O6-guanine; N7-guanine and N3-adenine).23 Temozolomide kills normal and cancerous cells yet is more harmful to rapidly dividing tumor cells.22 A newer modality in non-invasive therapy, tumor treating fields (TTFields), uses alternating electrical fields of intermediate frequencies to pulse through the scalp, disrupt tumor cell division, or cause tumor cell death.24 The avoidance of radiotherapy eliminates the risk of radiation necrosis and neuroinflammation linked to brain damage and cognitive impairment.25
Affect of Glioblastoma on the Parietal Lobe
The parietal neurons contribute to sensory spatial perception.13 Neuronal activity in the parietal lobe integrates multiple streams of sensory data to process the proximal relationship of different body parts to each other, orient the body’s relationship to other objects in space, and orient extrinsic objects to each other in the extra personal space.15 The parietal region also analyzes space using sensory modalities, specifies spatial targets for the motor system, generates attention, and analyzes visual motion.14
Anatomically, the parietal lobe is divided into the post-central gyrus, the supramarginal gyrus, the angular gyrus, and the superior parietal lobule on the outer hemisphere surface.26 The supramarginal and angular gyri comprise heteromodal cortex neurons receiving convergent inputs from other cortex regions dedicated to processing sensory modalities.15 Specifically, the sensory data from visual system projections such as the dorsal visuofugal pathway, in which a combination of retinotopic and visuomotor information results in the computation of events within the extra personal space.14 The sensory deficits experienced after tumor resection can be localized to exact locations within the parietal lobe. Neglect, or the propensity to ignore objects in the half of space opposite the side of the lesion, is a behavioral consequence observed after the development of parietal cortex lesions.13
Neurons in the intraparietal sulcus respond to visual stimuli and memory-guided eye movements.25 With difficulty locating and turning on a light switch, the subject in this case study appeared to experience deficits in the parieto-occipital sulcus extrastriate areas V6 and V6A for functional visuomotor coordination.27 In addition to describing a deficit of spatially directed reaching arm movements, the subject had an impaired response to visual stimuli and memory-guided eye movements, signaling intraparietal sulcus damage.28
Conclusion
Glioblastomas are aggressive tumors that cause symptoms early in their development, and with prompt identification, survival time can increase. Surgery was performed four days after the optometry visit, and the patient suffered low postoperative morbidity. Although elderly, he responded well to the adjuvant chemotherapy. The tumor resection impacted his sensory and motor function; nevertheless, the rapid neurology response enabled him to survive with the best possible quality of life. No identifiable health information was included in this case report.
Take home points
-
Glioblastoma is mitotically active with a growth rate of 1.4 percent per day and a volume doubling time of 49.6 days.
-
Subjective symptoms of visual disturbances in the form of hallucinations or visual field loss prompt an early diagnosis and lesion localization.
-
Parietal lobe glioblastoma surgical resection contributes to neuronal disruption, impairing neurosensory function.




.jpg)




.jpg)

