INTRODUCTION
Posterior radiation optic neuropathy is a rare complication of radiation treatment for head and neck tumors. At guideline-based radiation levels, the incidence of this complication is 1%-3% and can occur anywhere between 3 weeks and 9 years after radiation treatment.1,2 This report discusses a case of posterior radiation optic neuropathy in a patient who had undergone hypofractionated Gamma Knife radiation for a pituitary macroadenoma 2 years prior to devastating vision loss. Uniquely, the amount of radiation was within guidelines, and proposed mechanisms will be discussed. No identifiable health information was included in this case report.
CASE REPORT
A White man aged 71 years presented to the eye clinic for his annual examination. He was followed for the past 10 years as a low-risk normal-tension glaucoma suspect. During this time frame, his optic nerves, visual fields, and optical coherence tomography (OCT) remained stable with a maximum pressure of 15 mmHg right eye and 14 mmHg left eye (Figures 1 and 2). Medical history was significant for morbid obesity, sleep apnea, and acromegaly, and he was on albuterol, ocreotide, and cabergoline.
Four years prior to this examination, he was diagnosed with a pituitary macroadenoma that resulted in hormonal complications of acromegaly, for which he was treated with ocreotide and cabergoline. Despite treatment, his insulin-like growth factor-1 and growth hormone laboratory test results remained elevated. The pituitary lesion did not exert a mass effect on the optic chiasm and therefore did not affect his optic nerves or visual fields (Figure 3A and 3B). Because of hormone-related symptoms, he consulted neurosurgery for consideration of transsphenoidal resection. At this time, he was 400 pounds with a body mass index of 55.8. He was deemed too high risk because of his morbid obesity and sleep apnea, and he was referred to neuroradiology. Hypofractionated Gamma Knife radiation was considered and elected for. Prior to surgery, all medicines for acromegaly were discontinued for 3 months. Resultantly, his insulin-like growth factor-1 and growth hormone levels increased. During surgery, he received a total radiation dose to the isocenters of 25 Gray, with 12 hypofractionated doses of 1.8 Gray. The perioperative magnetic resonance images and radiation maps were not able to be obtained, but postsurgical notes indicate that these images were poor quality because of the use of flex coils to accommodate the patient’s size and an ill-fitting stereotactic head frame. After the surgery, medications remained unchanged, as did medical history. One month later, he resumed previous medications for acromegaly.
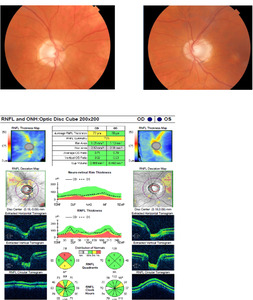
At that eye examination (now 2 years after radiotherapy), preliminary testing and anterior and posterior ocular evaluations were normal. There was also no visual field loss (Figure 2). Ten weeks later, the patient returned with right vision loss followed by vision loss in his left eye, which started 8 weeks prior. Visual acuity was no light perception in the right eye and 20/60 in the left eye. Preliminary tests confirmed an afferent pupillary defect in the right eye, and posterior pole findings revealed pallid nerves in both eyes (Figure 4). OCT retinal nerve fiber layer measurements revealed significant thinning compared with baseline (Figure 4). The 30-2 Humphrey visual fields revealed a new complete junctional scotoma (Figure 5). He denied any headache, nausea, diplopia, jaw claudication, weight loss, or fatigue symptoms. The patient was referred for urgent magnetic resonance imaging of the orbits, chiasm, and brain, and neurosurgery was paged. Initial imaging revealed no change to the macroadenoma from prior studies following his radiation treatment and no mass effect of the remaining tumor on the optic chiasm (Figure 3). The initial radiology interpretation indicated that there was no contrast enhancement of the visual pathway on T1 images. This excluded the differential diagnoses of macroadenoma regrowth, pituitary apoplexy, or other compressive lesions of the optic chiasm or optic nerves. Insulin-like growth factor-1 and growth hormone levels were normal. This prompted an evaluation for new onset optic nerve atrophy. Giant cell arteritis was excluded by blood work, including a complete blood count, erythrocyte sedimentation rate, C-reactive protein, as well as a temporal artery biopsy. All were normal. Further laboratory tests included Syphilis immunoglobulin G, Lyme serology, antinuclear antibody, vitamin B12, folate, QuantiFERON, and angiotensin converting enzyme. A computed tomography scan of the lungs was also ordered, as well as a lumbar puncture. Cerebral spinal fluid analysis was normal to include cell count, glucose, chloride, glutamine, lactate dehydrogenase, opening pressure, aquaporin-4 immunoglobulin G, and VDRL test. There were no myelin basic proteins found, no oligoclonal bands, and no cancerous, fungal, or bacterial cells present. All additional tests returned normal and without suspected pathology. At this time, the diagnosis was presumed radiation-induced optic neuropathy and neurosurgery ordered a series of 4 repeat contrast-enhanced magnetic resonance imaging over the following 3 weeks to evaluate for any potential macroadenoma growth, which all returned without a change in mass (Figure 3). However, there was abnormal enhancement on contrast-enhanced T1 images that began initially on the right optic nerve and subsequently spread to the optic chiasm and to both optic tracts (Figure 3), confirming posterior radiation-induced optic neuropathy. Of note, even though the initial imaging report (after the onset of visual symptoms) indicated that there was no enhancement along the visual pathway, a meeting with a multispecialty board years after his diagnosis did verify subtle enhancement in the right optic nerve near the chiasm in imaging immediately following his vision loss (Figure 3).
DISCUSSION
The pituitary gland regulates and secretes several endocrine hormones in the human body, including thyroid-stimulating hormone, growth hormone, luteinizing hormone, adrenocorticotropin, prolactin, follicle-stimulating hormone, oxytocin, and vasopressin.1 Pituitary adenomas make up 10%-20% of all intracranial tumors and can be functional, meaning they secrete hormones, or nonfunctional, as they do not secrete hormones.2 Functional pituitary adenomas may result in hyperpituitarism, in which at least 1 pituitary hormone is secreted in excess, or hypopituitarism, in which at least 1 pituitary hormone is reduced.2 Pituitary adenomas can also exert mass effects, depending on the level of compression on adjacent structures. Mass effects on the chiasm can result in visual field defects and reduced vision. Additional symptoms that may be found in an eye examination of ptosis, diplopia, mydriasis, anisocoria, or facial pain may occur if there is a mass effect on the cavernous sinus, whereas a mass effect on the dura can result in headache.3 The patient in this case experienced a growth hormone–secreting pituitary macroadenoma that resulted in acromegaly without mass effect on nearby structures. Acromegaly is confirmed by high levels of growth hormone and insulin-like growth factor-1 in blood serum tests. Patients with acromegaly experience enlarged hands, feet, jaw, and forehead. They may also experience overgrowth of joints and cartilage, as well as enlarged organs, and experience higher rates of sleep apnea, carpal tunnel syndrome, hypertension, cardiomyopathy, congestive heart failure, and diabetes.3
The primary goal of treating a growth hormone–producing pituitary adenoma (that is not causing mass effect complications) is to achieve hormone normalization.4 The first line of treatment is transsphenoidal resection, but many patients rely on multiple treatment options for long-term management.5 If surgical resection fails or is contraindicated, then medical management or radiotherapy may be offered.4,5 Medications for acromegaly include cabergoline, bromocriptine, ocreotide, and lancreotide.5 For the patient in this case, his weight and sleep apnea placed him at too high of a risk for surgical resection. He was treated with ocreotide and cabergoline for 3 years, but his acromegaly hormone levels remained elevated. Since medical treatment was unsuccessful, he was referred to neuroradiology who then completed stereotactic Gamma Knife radiotherapy. This surgery allows a high amount of radiation to be delivered to a small target, with a steep gradient at the tumor margins, thus reducing the risk of radiation damage to nearby structures.5,6 Gamma Knife radiotherapy for pituitary adenomas is typically administered with several smaller doses, termed hypofractionated therapy.6 The patient in this case received 12 hypofractionated doses of radiation applied to his pituitary macroadenoma.
Radiotherapy is considered successful when growth hormone levels drop to 50% of their preradiotherapy levels while off medications. The patient in this case remained on his medications, but his hormone levels dropped to 50% of their preradiotherapy levels 2 years after surgery. The most common complication of radiotherapy is hypopituitarism, which ranges in incidence from 0%-40%.4,6 The incidence of radiation-induced optic neuropathy for those with growth hormone secreting pituitary adenoma treated by radiotherapy ranges from 0-5%.6
Radiation-induced optic neuropathy may occur anywhere from 3 weeks to 9 years after the initial radiation treatment, with the vast majority developing within 3 years.7,8 Radiation-induced optic neuropathy presents with acute, painless loss of vision in one or both eyes, concurrently or successively.7,8 After the initial rapid loss of vision, subsequent vision loss can occur over weeks. Vision loss can vary extensively, but up to 85% of those affected experience acuities of 20/200 or worse, and 45% experience no light–perception vision.8 When radiation necrosis appears anterior to the lamina cribosa, this results in an acutely swollen optic nerve that eventually develops into pallor. This is termed anterior radiation-induced optic neuropathy and occurs secondary to irradiated intraocular, orbital, or paranasal tumors.7,8 Posterior radiation-induced optic neuropathy, conversely, presents with a normal-appearing nerve that eventually turns pale because the area of necrosis occurs behind the lamina cribosa. It can occur with irradiated head and neck tumors but more commonly with brain tumors near the visual pathway or the cavernous sinus.7 For all radiation-induced optic neuropathy, pallor develops 6 to 8 weeks after the acute event. Accompanied radiation-induced optic neuropathy–associated visual field defects can show any form of chiasmal or optic nerve defects, depending on the location of necrosis.8
Prior to radiation treatment, so-called organs at risk are extensively imaged to avoid unintended radiation damage. For this patient, the optic chiasm and optic nerves were accordingly identified. The recommended safe total level of radiation is <55 Gray, with fractionated doses preferably of ≤1.8 Gray.7 Additionally, the total amount of radiation recommended close to the visual pathway margin is ≤8 Gray.4,8 Because of the susceptibility of these organs at risk to radiation damage, Gamma Knife radiation is only recommended for tumors that are 3 mm to 5 mm away from the optic chiasm.4 When surgeons follow these guidelines, the risk of development of radiation-induced optic neuropathy is 1%-3%. However, when total radiation dose is >60 Gray, the risk of radiation-induced optic neuropathy increases to 20%.1,4 In this report, the patient received a total radiation dose of 25 Gray to the isocenters within the body of the tumor, completed with 12 hypofractionated doses of 1.8 Gray. The total radiation dose administered close to the visual apparatus was 8 Gray, and the macroadenoma was spaced 5 mm away from the optic chiasm. As such, all treatment levels fell well within recommended guidelines. Notably, the largest risk factor to developing posterior radiation-induced optic neuropathy is the total amount of radiation received, and the 25 Gray this patient received was far less than the 55 Gray recommended safety dosage.7
There are several theories why this patient developed this complication, despite receiving radiation within recommended safety guidelines. First, this patient was severely overweight, which is known to make precise imaging difficult, and surgical notes confirmed that perioperative imaging was of reduced quality.8 This could have resulted in measurement error if the organs at risk were not mapped appropriately, or if the adenoma was closer to the visual pathway than originally thought. In addition, this patient had acromegaly, which results in larger skull air sinuses.8 This may have resulted in a higher-than-intended level of radiation to the optic chiasm, although the posited 7% error would still place the patient at safe levels.8,9 Furthermore, it is theorized hormone imbalances caused by acromegaly can increase vulnerability to radiation damage in the visual pathway, but this is not widely accepted.2,10 Other risk factors (not pertinent to this patient) for radiation-induced optic neuropathy include reirradiation treatment, preexisting tumor compression of the optic nerve and chiasm,8 hypertension, and diabetes.11
The diagnosis of posterior radiation-induced optic neuropathy is based on clinical appearance, a history of radiation surgery, and imaging. It should be considered a diagnosis of exclusion, after other infectious, inflammatory, compressive, and vascular causes of optic nerve pallor or edema. In this case, the patient presented with sudden vision loss and a complete junctional scotoma, suggesting involvement of the optic chiasm and right optic nerve. The primary differential diagnoses initially excluded were a pituitary tumor recurrence or progression, a pituitary apoplexy, and any other compressive lesion of the optic chiasm or optic nerves.7,8,12 A pituitary apoplexy occurs when there is a sudden hemorrhage or infarction of the pituitary gland.8 In this case, MRI revealed no pituitary adenoma progression, no mass effect on the optic chiasm or optic nerves, and no infarction or hemorrhage within the pituitary gland. Toxic optic neuropathy was excluded by history and a review of his past and present medications. To exclude other causes of suspected retrobulbar optic nerve injury, a workup was completed, including an evaluation for giant cell arteritis, sarcoidosis, syphilis, tuberculosis, collagen vascular diseases, Lyme disease, nutritional deficiencies, and optic neuritis. Ischemic chiasmopathy, or loss of blood flow to the chiasm from the circle of Willis, was also considered, but this is more common after cardiac surgeries or procedures to the internal carotid arteries. It was also less likely, as imaging did not reveal any emboli or significant loss of blood flow to the chiasm and conversely showed enhancement.13
At this point, it was concluded that the patient likely had posterior radiation necrosis of the optic nerve and chiasm, despite receiving safe radiation levels. Approximately 3 weeks after his visual symptoms began, repeat magnetic resonance imaging confirmed the diagnosis of radiation-induced optic neuropathy, as his T1 contrast-enhanced images revealed an abnormal enhancement along the right optic nerve, optic chiasm, and both optic tracts. A review of this case with a multispecialty board years after his diagnosis, indicated that subtle enhancement of the right optic nerve was also present during his first imaging following his vision loss. Hudgins et al recommends all patients with suspected posterior radiation optic neuropathy receive gadolinium-enhanced, fat-suppressed T1 weighted images of the optic nerve and chiasm, as unenhanced T1 and T2 images will look unremarkable.14 This nonspecific enhancement can be found along the affected segment, but it is often difficult to capture and therefore cannot be relied on to make a diagnosis of posterior radiation-induced optic neuropathy.8 Such enhancement may present from 2 to 17 months after the initial vision loss and can be transient.8 In addition, the technique needed to visualize this change is specific, as lack of fat suppression or saturation techniques can cause this enhancement to not be visualized.14 This case highlights how difficult it can be to visualize this enhancement, thus leading to a difficult or delayed confirmed diagnosis of radiation-induced optic neuropathy.
There are no randomized, masked, double-blind studies evaluating treatment for posterior radiation-induced optic neuropathy, and thus there currently is a lack of evidence-based intervention. Theoretical medical treatments, with some published reports, include systemic corticosteroids, anticoagulants, and vasodilators. However, studies and case reports have not shown consistent promise for these medications, and their use is not recommended because of potential side effects and cost.7,8,12,15 Alternatively, cases of bevacizumab (a monoclonal vascular endothelial growth factor antibody) have shown varied success, with some indicating visual and radiographic improvement after treatment15–19 and others demonstrating no positive outcome after treatment.20 The most widely recommended treatment to consider, albeit with caution, is hyperbaric oxygen.7,8,15 Levy et al, in a review of literature and retrospective case studies, found scattered cases of improved acuity after treatment with hyperbaric oxygen but only if treatment was initiated within 48 hours of initial acuity loss and prior to the development of optic nerve pallor. Thus, he concluded that although hyperbaric oxygen is relatively safe, treatment is costly, laborious, and more likely to result in false hope.21 Although some suggest prompt treatment with hyperbaric oxygen or intravenous bevacizumab,7 others suggest a more conservative approach, forewarning clinicians of the potential harm that could result until higher-level research studies are conducted.22 Overall, the best treatment for posterior radiation-induced optic neuropathy is prevention to ensure that radiation treatment is administered at safe recommended levels, as well as visual rehabilitation, until further research is conducted on intravenous bevacizumab and hyperbaric oxygen.
CONCLUSION
Posterior radiation optic neuropathy is a rare but devastating complication of radiation to head and neck tumors. Because of the wide range of time following radiation to presentation, diagnosis can be challenging, and efforts should be made towards a diagnosis of exclusion. The primary diagnosis to exclude is a recurrence or growth of the irradiated tumor, followed by other causes of vascular, inflammatory, and infectious causes of optic nerve edema and/or pallor. Appropriate imaging should be ordered, primarily to evaluate for any compressive cause but also to possibly demonstrate nonspecific enhancements supporting the diagnosis of radiation optic neuropathy. Even when radiation is applied within recommended safety guidelines, this complication may still occur, as it did in this case. Currently, there is no standard recognized treatment for radiation-induced optic neuropathy, so efforts should be directed towards prevention strategies. Case reports on treatment modalities exist, but there is not enough conclusive evidence at this time for these therapies to be recommended.
TAKE HOME POINTS
-
Radiation-induced optic neuropathy can be difficult to diagnose, as symptoms of vision loss may start within weeks or years after initial radiation treatment.
-
This is a diagnosis of exclusion after other causes of optic neuropathy, such as inflammatory, compressive, infectious, and vascular causes have been ruled out.
-
Imaging can ultimately be helpful in solidifying the diagnosis, although subtle findings can easily be missed.
-
If preventative measures fail, low vision rehabilitation should be considered.


_and_pituitary_adenoma_(green_.png)
.png)




_and_pituitary_adenoma_(green_.png)
.png)

