Introduction
Neurotrophic keratitis is a rare degenerative corneal disease caused by decreased or absent corneal sensory innervation, which subsequently leads to epithelial breakdown, delayed corneal healing, and eventually corneal ulceration, melting, and perforation.1 The loss of corneal sensory innervation can have many different etiologies, depending on the location of the lesion. The severity of neurotrophic keratitis is most often graded based on the Mackie classification with 3 stages.2 Recently, a consensus panel proposed a new classification system with 6 stages (Neurotrophic Keratopathy Study Group Classification).3
This case describes a patient experiencing unilateral neurotrophic keratitis caused by acoustic neuroma or the surgical effect of removing the tumor. She was fitted with a scleral lens in the right eye. After 1 year of successful lens wear, she now has a healthier ocular surface and enjoys the resulting better visual outcome. A general overview of the disease with emphasis on novel treatment strategies is also discussed. No identifiable health information was included in this case report.
CASE REPORT
Initial Visit
A White woman aged 57 years presented to the clinic for scleral lens fitting with a referral from her corneal specialist. At the initial evaluation on July 7, 2020, she complained of constant blurred vision at distance and near, with and without correction in the right eye, for 10 years. She denied pain, dryness, or irritation. Her ocular history was significant for the prior diagnoses of neurotropic keratitis in the right eye. She had no prior history of contact lenses wear and was only using over the counter +2.50 reading glasses. At the time of the initial visit, she was taking prednisolone acetate ophthalmic suspension 3 times a day, erythromycin ophthalmic ointment every night, and Restasis once a day in the right eye.
Her medical history was significant for acoustic neuroma, breast cancer in remission, hypercholesterolemia, and hypertension. The acoustic neuroma was surgically excised 10 years ago, before the onset of the complaint of blurred vision in the right eye. The referring corneal specialist determined that acoustic neuroma was the cause of neurotropic keratitis.
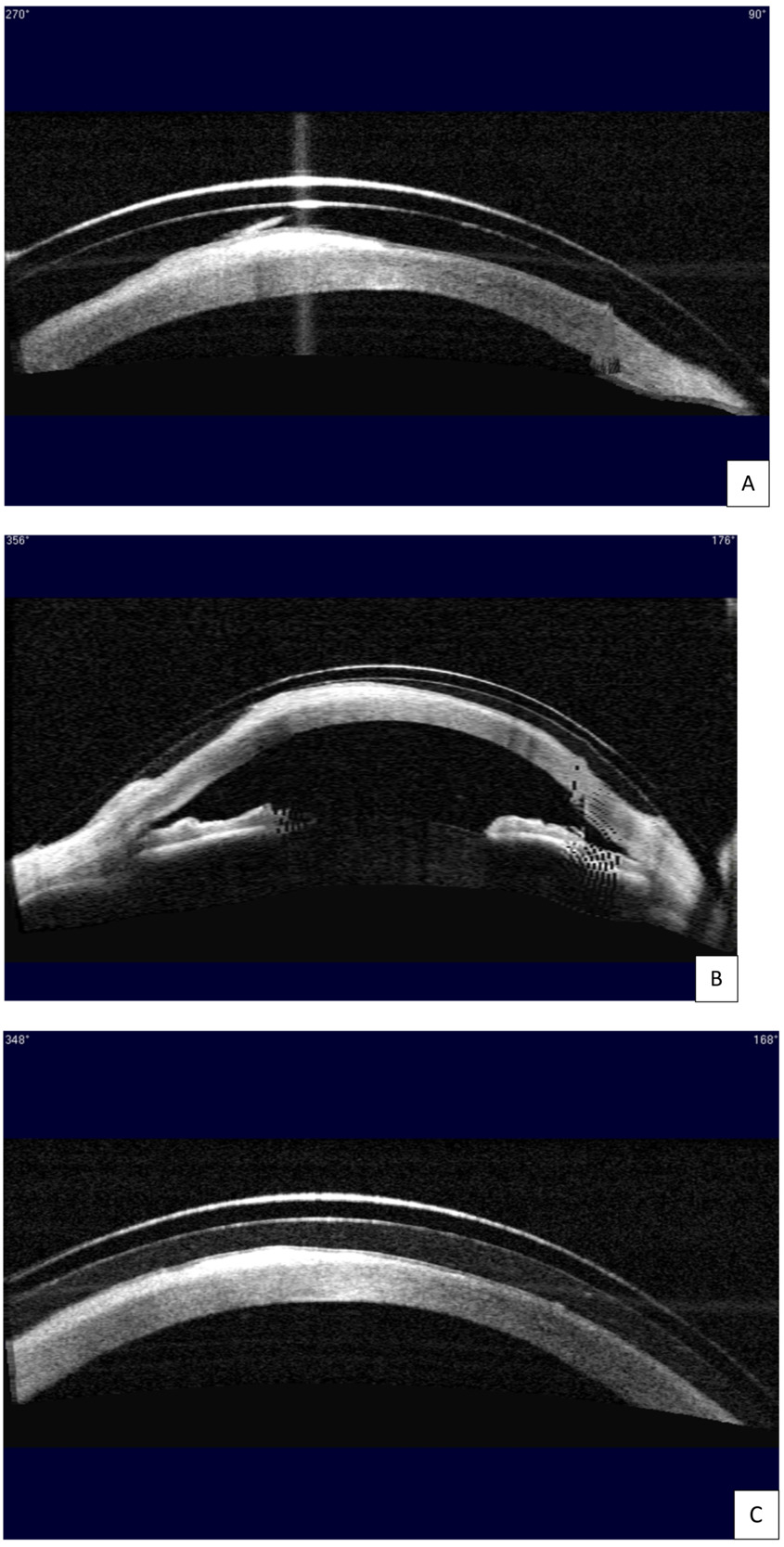
At the initial evaluation, she presented with a distance best corrected visual acuity of 20/60- in the right eye and 20/20 in the left eye with a refraction of -1.25 - 0.50 x 065 right eye and +1.00 sphere left eye. Near visual acuity was 20/50 right eye and 20/20 left eye with +2.50 additional power over the manifest refraction. Slit lamp examination of the anterior segment revealed a large area of horizontal corneal scar tissue across the visual axis with corneal neovascularization temporally in the right eye. The area of the scar tissue stained with sodium fluorescein, indicating active epithelial defects (Figure 1A). Fluoroimaging using the OCULUS Keratograph 5M highlighted the extensive epithelial breakdown over the corneal scar and revealed diffuse 2+ superficial punctate keratitis across the entire corneal surface, more pronounced in the interpalpebral zone (Figure 2A). A few large epithelial defects were oval shaped. Using the Zeiss Visante anterior segment optical coherence tomography, cross-section images of the cornea at all angles confirmed extensive epithelial but minimal stromal involvement in the corneal scar tissue. An example of a corneal cross-section image through the scar tissue is shown in Figure 3A.
At the initial evaluation, the severity of neurotrophic keratitis was graded to be stage II based on the Mackie classification2 and stage 4 based on the Neurotrophic Keratitis Study Group classification,3 since there were oval-shaped epithelial defects present and minimal stromal involvement with no signs of acute corneal ulceration (Figure 2A and Figure 3A).
The right eye was fit with a scleral lens with the goal of improving her overall visual function. Onefit MED (CooperVision Specialty EyeCare – Blanchard) was chosen for its highly customizable parameters for fitting irregular corneas and its thin lens profile that would maximize oxygen transmission. Several diagnostic lenses were trialed on the eye. According to guidelines provided by the laboratory, the ideal diagnostic lens was chosen with a sagittal height that would lead to a central fluid reservoir thickness of ~350 μm immediately on insertion.4 The ideal diagnostic lens had the following parameters: 4400 sagittal height / prolate shape / 15.6 diameter / -1.50 power / 0.25 central thickness / standard midperipheral value / standard limbal value / +75/-75 edge value. Clearance assessment after 4 hours of wear time with anterior segment optical coherence tomography showed ~300-μm central fluid reservoir thickness and ~40-μm fluid reservoir thickness over the epithelial defects and the scar tissue (Figure 3A). Slit lamp evaluation revealed good centration and coverage of the lens without edge lift, compression, or blanching 360. There was an overrefraction of +3.75 sphere with best corrected visual acuity of 20/30. The fit and the vision were deemed acceptable, and the first lens was ordered with the following parameters: 4400 / 15.6 / +2.25 / 0.23 / standard midperipheral value / standard limbal value / +75/-75 edge value /Optimum Infinite material.
To ensure the long-term health of the already compromised ocular surface, the lens was designed to maximize oxygen transmission to the cornea through the scleral lens and the postlens tear reservoir. The lens was made with a material of high oxygen permeability (Optimum Infinite with 180 Dk) and minimal thickness. Fluid reservoir thickness would be minimized in the final lens (<300 μm centrally) while avoiding corneal touch.
First Dispense Visit and First Follow-up Visit
At the first dispense visit on July 15, 2020, the lens fit well on insertion, and the patient had a best corrected visual acuity of 20/30 and no overrefraction in the right eye. The lens was dispensed to her after insertion and removal training. She was instructed to instill medicated eye drops in the right eye without the scleral lens to prevent solution toxicity. She was advised to take off the lens midday to instill the prednisolone acetate ophthalmic suspension and to wait 15 minutes after drop instillation before reinserting the right scleral lens.
At the first follow-up visit on July 21, 2020, her best corrected visual acuity was 20/25 with plano overrefraction in the right eye. Slit lamp assessment continued to show a satisfactory fit. However, clearance assessment with the anterior segment optical coherence tomography revealed an area of touch at 356°, indicating insufficient clearance (Figure 3B). The lack of clearance may cause corneal abrasion and compromise the health of the corneal surface. Therefore, the sagittal height of the scleral lens was raised by 100 μm, and the second right lens with the following parameters was ordered: 4500 / 15.6 / +1.12 / 0.22 / standard midperipheral value / standard limbal value / +75/-75 edge value / Optimum Infinite material.
Second Dispense/Follow-up Visit
At the second dispense and follow-up visit on August 3, 2020, the patient had 20/25 best corrected visual acuity with plano overrefraction in the right eye, and an excellent fit was observed with both slit lamp examination and clearance assessment with the anterior segment optical coherence tomography after 4 hours of wear time. The second right lens was dispensed. She was instructed to come back for follow-up every 6 months or sooner if issues occur.
1-Year Follow-up
The patient has been wearing the right scleral lens successfully since the August 03, 2020 visit. At the 1-year follow-up on December 22, 2021, she again presented with best corrected visual acuity of 20/25 with no overrefraction in the right eye. She reported clear and comfortable vision all day with scleral lens wear. Clearance assessment with the anterior segment optical coherence tomography showed no touch 360°, with a central fluid reservoir thickness of ~250 μm after 6 hours of wear time (Figure 3C). Slit lamp examination continued to show an excellent-fit relationship. The most striking finding is that 1 year of scleral lens wear significantly improved the severity of neurotrophic keratitis in this patient. When comparing the anterior segment photo on December 22, 2021 (Figure 1B), to the initial photo on July 7, 2020 (Figure 1A), significantly less corneal haze was seen in the horizontal scar tissue across the visual axis, except in a small area inferonasally over the pupil and away from the line of sight at the end of the temporal corneal neovascularization. Moreover, when comparing the fluoroimage on December 22, 2021 (Figure 2B), to the initial image on July 7, 2020 (Figure 2A), all oval-shaped epithelial defects have healed, and only 1+ diffuse superficial punctate keratitis was present on the corneal surface. From July 7, 2020, to December 22, 2021, the severity of her neurotrophic keratitis had improved from stage 2 to stage 1 based on the Mackie classification2 and from stage 4 to stage 2 based on the Neurotrophic Keratitis Study Group classification.3
At her next appointment with her corneal specialist in February 2022, seeing that the severity of neurotrophic keratitis had significantly reduced, the corneal specialist discontinued prednisolone acetate ophthalmic suspension and erythromycin ophthalmic ointment for the right eye. She is currently only using Restasis once a day in the right eye.
DISCUSSION
In neurotrophic keratitis, the loss of corneal sensory innervation can have many different etiologies. The trigeminal nerve (cranial nerve V) branches to form the ophthalmic nerve, whose nasociliary branch provides sensory innervation to the cornea via the long and short ciliary nerves.5 Disruption along any step of this pathway may lead to impaired corneal sensory innervation and, therefore, neurotrophic keratitis. Neurotrophic keratitis is most commonly caused by direct damages to the corneal nerve endings.6 Examples include herpes keratitis, contact lens abuse, chronic topical drug toxicity, and corneal surgery.6 Procedures that affect other innervations of the long and short ciliary nerves may also affect the corneal nerve endings, as neurotrophic keratitis has been reported after vitrectomy or laser for proliferative diabetic retinopathy.7,8 Lastly, conditions that affect cranial nerve V may lead to neurotrophic keratitis. Examples include intracranial masses (eg, acoustic neuroma), aneurysms, diabetes and multiple sclerosis.9 For the patient in this case report, the cause of neurotrophic keratitis is acoustic neuroma—a rare, benign, and slow-growing growth that develops on the vestibulocochlear nerve (cranial nerve VIII), which can enlarge to affect the nearby cranial nerves, including cranial nerve V and VII.10 Besides ocular manifestations, there can be significant neurological manifestations, such as hearing loss, facial paresis, and dizziness.10
In the literature, the goals of neurotrophic keratitis therapy are to promote corneal healing and to prevent corneal ulceration and perforation.11 The general management of neurotrophic keratitis shares many similarities with the general management of other ocular surface diseases, such as dry eye syndrome and exposure keratitis.12 Mackie stage 1 neurotrophic keratitis is managed with the frequent use of preservative-free artificial tears and lubricating ointment, punctal plugs, autologous serum tears, and amniotic membranes.11 At stage 2, additional treatments and procedures are used, such as daily antibiotic eye drop or ointment to prevent bacterial infections, daily topical corticosteroids to control inflammation, botulinum-induced ptosis, and tarsorrhaphy.1,12 At stage 3, when corneal ulceration and melting occur, N-acetylcysteine, oral tetracycline and medroxyprogesterone can be prescribed.1 When corneal perforation occurs, small perforations are treated with cyanoacrylate glue, bandage contact lens, or amniotic membrane; larger perforations are treated with penetrating or lamellar keratoplasty.1,12
The patient was taking prednisolone acetate ophthalmic suspension 3 times a day at the beginning of the scleral lens fitting process. This was part of a pulsed steroid course with a slow taper initiated by her corneal specialist to control ocular surface inflammation. The use of steroid therapy in an eye with neurotrophic keratitis is controversial, as it may increase the risk of corneal melting.13 Furthermore, the combination of steroid eye drops and contact lens wear may also increase the risk of infectious keratitis.14 An alternative would be to initiate scleral lens wear after the steroid course is completed. After communicating with the corneal specialist, it was determined that she could start wearing the scleral lens while taking the steroid eye drops, given that she is monitored closely and only inserts the scleral lens 15 minutes after drop instillation.
The typical recommended dosing for Restasis is twice daily; however, one study found that after 1 year of following the recommended dosing, signs of dry eye disease did not worsen after lowering the dosing from twice daily to once daily.15 Thus, the patient’s corneal specialist lowered the dosing in the right eye after 2 years of following the twice-daily dosing when she expressed concerns for the cost of the Restasis medication.
Several novel treatments of neurotrophic keratitis have emerged in the recent years. Firstly, cenegermin (OXERVATE; L’Aquila, Italy) is a topical medication that contains recombinant human nerve growth factor. Two large randomized controlled trials recruited patients with stage 2 or 3 neurotrophic keratitis, and the results showed that the cenegermin group demonstrated significant improvement in corneal signs or even complete corneal healing compared with the control group.16,17 OXERVATE received US Food and Drug Administration approval in the United States for the treatment of stage 2 or 3 neurotrophic keratitis in 2018.17 Secondly, corneal neurotization is a promising surgical technique for the treatment of neurotrophic keratitis.18 Corneal neurotization involves grafting a segment of another sensory nerve (eg, supraorbital or suprotrochlear nerve) into the cornea, with preliminary results showing improved corneal sensation and visual acuity postsurgery.18,19 Other investigational treatments for neurotrophic keratitis include ReGeneraTing Agents,20 topical thymosin β4,21 combined-substance P-derived peptide and insulin-like growth factor 1,22 varenicline nasal spray,23 and other topical recombinant growth factors.24
In the current case report, the severity of neurotrophic keratitis and best corrected visual acuity in the right eye were both significantly improved 1 year after the initiation of scleral lens wear. The patient was briefly prescribed OXERVATE for a month by her corneal specialist at the beginning of 2021. However, the drop was discontinued after 1 month because of intolerance to the medication, as she experienced severe conjunctival hyperemia in the right eye. Therefore, scleral lens wear alone likely contributed to the corneal healing process. Scleral lenses have been proposed as a novel treatment and management strategy for neurotrophic keratitis.25 Scleral lenses play an important role in ocular surface disease management by enclosing the cornea in a moist environment made of saline and tears.26 The fluid reservoir is thought to protect the cornea against outside forces and to promote corneal healing.26 Furthermore, given the improvement in the severity of neurotrophic keratitis, she was able to discontinue the daily use of prednisolone acetate ophthalmic suspension and erythromycin ophthalmic ointment in the right eye. By reducing the total number of ocular medications, a less burdensome treatment plan improved her quality of life. Overall, the current case report supports adding scleral lens to the treatment protocol for the early to moderate stages of neurotrophic keratitis because of its remarkable corneal healing effect and its potential for reduced treatment burden.
To our knowledge, no randomized control trials have been conducted on the effectiveness of scleral lens wear as a treatment for neurotrophic keratitis, but 2 individual case reports27,28 and 1 larger case series (Alshami S, et al. IOVS 2018;59:ARVO E-Abstract 1795) involving 24 eyes have reported promising treatment outcomes. All the case reports showed that scleral lens wear healed previously nonhealing epithelial defects, which led to significantly improved visual acuity and quality of life (Alshami S, et al. IOVS 2018;59:ARVO E-Abstract 1795).27,28 Future studies, especially large randomized control trials, are needed in establishing scleral lenses as a standard-of-care treatment option for neurotrophic keratitis.
CONCLUSION
Overall, the current case report adds to the body of evidence that scleral lenses are an effective non-invasive treatment option for neurotrophic keratitis. Scleral lens wear promotes corneal healing and may lead to improved visual acuity and fewer ocular medications, all of which contribute to improved overall quality of life for neurotrophic keratitis patients. Therefore, scleral lens fitting may be suggested to patients with neurotrophic keratitis at early to moderate stages of the disease.
No identifiable health information was included in this case report.
Take Home Points
-
Recent advances in neurotrophic keratitis research include a new grading scale and several novel treatment strategies.
-
Scleral lenses are an effective non-invasive treatment option for neurotrophic keratitis with the benefits of visual rehabilitation, corneal healing and reduced treatment burden.
-
Scleral lens fitting may be suggested at early to moderate disease stages.

_at_the_initial_visit_on_july_7__2020__th.png)
_at_the_initial_visit_on_july_7__2020__four_oval-shaped_.png)

_at_the_initial_visit_on_july_7__2020__th.png)
_at_the_initial_visit_on_july_7__2020__four_oval-shaped_.png)