Introduction
Central nervous system hemangioblastomas are benign, slow-growing vascular neoplasms and represent approximately 2% of intracranial tumors and 7% of posterior fossa tumors, making them the most common primary intra-axial tumor.1–6 Central nervous system hemangioblastomas are characteristically very vascular structures and tend to arise infratentorially.5 They have specifically been shown to most commonly occur around the cerebellum and fourth ventricle (76%). Approximately 9% of central nervous system hemangioblastomas arise from the cerebral hemispheres, whereas 7% arise from the spinal cord. Of the spinal cord hemangioblastomas, thoracic hemangioblastomas are most common.3,7 Only 5% of central nervous system hemangioblastomas arise from the brainstem. On neuroimaging, hemangioblastomas are often solid, solid-cystic, or mainly cystic masses with solid enhancing nodule; there can be internal and peripheral feeding vessels.5
Case Report
Initial Examination
An 18-year-old man presented for initial eye examination without ocular or visual complaints. He had a history of acid reflux, with associated emesis, which started 2 years prior. He had several unrevealing abdominal ultrasounds. His symptoms were somewhat improved by famotidine. Within the last few months leading up to this examination, he reported difficulty swallowing and was only eating soft foods. He reported associated weight loss. These symptoms continued to be attributed to a gastroenterologic issue.
On examination, the patient’s best-corrected visual acuity was 20/20 in the right eye and 20/20 in the left eye. Confrontation fields were full to finger counting in both eyes, although an enlarged blind spot was questioned. Pupils demonstrated physiologic anisocoria with no evidence of an afferent pupillary defect. Color vision was normal in both eyes. Ocular motilities were full in both eyes and cover testing revealed only a small, two prism diopter, comitant esophoria. He demonstrated fusion with Worth-4-dot testing.
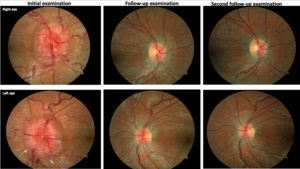
Anterior segment health was unremarkable. Intraocular pressures were 17 mm Hg in each eye. Posterior segment examination revealed significantly edematous and hyperemic optic discs with vessel obscuration, temporal Paton’s lines, and loss of the physiologic cup bilaterally (Figure 1). Ancillary testing, including optical coherence tomography, Humphrey visual fields, and fundus photos, was performed. Optical coherence tomography of the optic nerve demonstrated an elevated neuroretinal rim and retinal nerve fiber layer of both eyes consistent with papilledema. Ganglion cell analysis revealed 360-degree thinning bilaterally with evidence of serous macular detachment in each eye (Figure 2). A 24-2 Humphrey visual field demonstrated an enlarged blind spot with superior central/cecocentral defect and apparent inferior nasal step in the right eye and enlarged blind spot with central/cecocentral defect in the left eye (Figure 3).
Cursory neurologic examination revealed abnormal function of the glossopharyngeal nerve (cranial nerve IX) and the vagus nerve (cranial nerve X) as evidenced by absent soft palate elevation, difficulty with swallowing (dysphagia), and abnormal speech (dysarthria). Poor tongue protrusion and tongue fasciculations indicated abnormal hypoglossal nerve (cranial nerve XII) function. The patient demonstrated mild finger-to-nose ataxia, ataxic gait, difficulty with tandem gait, and a positive Romberg sign concerning for cerebellar dysfunction.
The combination of bilateral optic disc edema, ataxia, and involvement of multiple lower cranial nerves was suspicious for a large fourth ventricular mass with medullary and cerebellar compression as well as obstructive hydrocephalus. The patient was immediately sent to the hospital emergency room where magnetic resonance imaging of the brain and orbits with contrast (Figure 4) demonstrated an enhancing solid and cystic mass centered within the floor of the fourth ventricle, with significant associated mass effect, including effacement of the medulla and fourth ventricle. The presence of panventricular enlargement confirmed obstructive hydrocephalus secondary to this posterior fossa mass. Magnetic resonance venography ruled out venous sinus thrombosis. Lumbar puncture was contraindicated secondary to the large posterior fossa mass. The next day he underwent suboccipital craniotomy with mass resection; pathology findings were consistent with hemangioblastoma.
Follow-up Examination
Two months later, while still undergoing outpatient rehabilitation, the patient denied balance issues and reported much improvement with only mild residual speech and swallowing difficulty. Best-corrected visual acuity was 20/20-1 in the right eye and 20/20-1 in the left eye. Repeat 24-2 Humphrey visual field testing demonstrated improvement in each eye, now with less blind spot enlargement, and less dense central/cecocentral and nasal defects in each eye as compared with the initial testing (Figure 3). All other afferent and efferent testing was unchanged and remained normal. Posterior segment examination demonstrated definitely improved, but still significant, residual optic disc edema bilaterally. Obscuration of the retinal vessels at the disc margin was still appreciated in both eyes, but Paton’s lines were now only present in the left eye. There was no evidence of a spontaneous venous pulsation in either eye. Small cupping was now visible in both eyes (Figure 1).
Updated optical coherence tomography of the optic nerve demonstrated residual, but significantly improved, neuroretinal rim and retinal nerve fiber layer elevation in both eyes. Ganglion cell analysis showed no evidence of thinning (Figure 2).
Cursory neurologic examination revealed the following abnormalities, which were all improved from the initial examination: dysfunction of cranial nerves IX, X, and XII, mild finger-to-nose ataxia, difficulty with tandem gait, and a positive Romberg test.
Second Follow-up Examination
After another 2 months, the patient reported complete resolution of symptoms. Best-corrected visual acuity was 20/20 in each eye. Repeat 24-2 Humphrey visual field testing showed continued improvement in blind spot enlargement, and residual central/cecocentral and nasal defects bilaterally (Figure 3). Afferent and efferent testing was otherwise unremarkable and stable. Undilated examination demonstrated optic discs with distinct margins, resolution of disc edema, and small cupping bilaterally. There was no longer any evidence of vessel obscurations or Paton’s lines in either eye (Figure 1). A spontaneous venous pulsation was now noted in the left eye, suggestive of normalized intracranial pressure. Optical coherence tomography of the optic nerve demonstrated almost complete resolution of neuroretinal rim and retinal nerve fiber layer elevation in both eyes. Ganglion cell analysis demonstrated borderline superior nasal thinning in the right eye and definite superior nasal thinning in the left eye (Figure 2). Both the optical coherence tomography and Humphrey visual field findings suggest possible mild postpapilledema atrophy secondary to chronic papilledema. Cursory neurologic examination demonstrated continued improvement, now only noting residual dysfunction of cranial nerve XII and mild finger-to-nose ataxia.
Discussion
Central nervous system hemangioblastomas tend to arise in the region of the fourth ventricle and have the capacity to cause severe neurologic deficits either by direct compression of nearby structures or by increasing intracranial pressure secondary to a restriction of cerebrospinal fluid outflow.8 Because they are slow growing with periods of arrested growth, symptoms can be attributed to other causes before a correct diagnosis is finally made. Prior to the patient’s eye examination, this patient’s symptoms were initially thought to be associated with acid reflux. An eye examination with cursory neurologic examination was critical in revealing not only papilledema, suggestive of increased intracranial pressure, but also cranial nerves’ IX, X, and XII involvement suggestive of medullary damage and ataxia suggestive of cerebellar damage. These findings, along with understanding the function of key anatomical structures, allowed accurate localization of the lesion, recognition of the emergent nature of the presentation, and selection of the appropriate care team to handle the patient’s care.
Papilledema can be defined as bilateral optic disc edema secondary to elevated intracranial pressure. High intracranial pressure may be idiopathic or may be secondary to a pathologic process including, but not limited to, intracranial mass, intracranial hemorrhage, or venous sinus thrombosis.9 Regardless of etiology, any increase in intracranial volume will result in increased intracranial pressure.9,10 As in this case, a tumor situated within the floor of the fourth ventricle would block drainage of cerebrospinal fluid, thereby causing obstructive hydrocephalus and associated enlarged ventricles, the most common neuroimaging finding in patients with central nervous system hemangioblastomas.1,11 Other common neuroimaging findings associated with increased intracranial pressure include posterior scleral flattening, optic nerve tortuosity, and enlarged optic nerve sheath diameter.12 Cerebrospinal fluid buildup within the optic nerve sheath is a precursor to the presence of bilateral optic disc edema on funduscopic examination.9
Patients with papilledema may initially demonstrate enlarged blind spots with visual field testing because of the enlarged size of the edematous optic disc. If left untreated, increased intracranial pressure has the potential to cause irreversible vision loss by way of direct compression of, and damage to, the axonal optic nerve fibers within the subarachnoid space. This increased pressure along the optic nerve axons disrupts or halts axoplasmic flow and causes intraneuronal ischemia.9
Other ocular signs related to increased intracranial pressure may include unilateral or bilateral abduction deficits. Increased intracranial pressure can cause compression of the abducens nerve as it runs over the petrous ridge of the temporal bone and through Dorello’s canal. This can cause impairment resulting in unilateral or bilateral abduction deficits with corresponding eso deviation on cover testing. There may be corresponding symptoms of double vision, particularly on lateral gazes.13
Once papilledema has been identified, it is critical to determine the cause. As seen in this case, cursory neurologic examination allowed us to identify several dysfunctional cranial nerves, which in the context of papilledema, allowed for localization of the lesion to the region of the fourth ventricle. Recall, cranial nerves IX, X, and XII are paired cranial nerves that arise from the nucleus ambiguous in the medulla oblongata of the brainstem.14 After exiting the medulla, their course through the cranial cavity is short. They exit the brainstem ventrally and pass through the cerebellopontine angle. Cranial nerves IX and X exit the skull via the jugular foramen, and cranial nerve XII exits the skull via the hypoglossal foramen. They then travel on to innervate structures involved in the execution of swallowing, taste, speech, heart rate, blood pressure control, and peristalsis. Dysfunction of these nerves characteristically results in dysphagia, dysphonia, dysarthria, and aspiration.15
Among the many functions of cranial nerve IX, it most notably supplies the stylopharyngeus muscle, allowing for elevation of the pharynx during swallowing and speech.14,15 If nonfunctional, patients will describe symptoms of dysphagia. Thorough history-taking allowed this symptom to be uncovered in this case; it had previously been attributed to gastroenterologic problems. Additionally, cranial nerve IX also acts in the stimulation of the upper pharynx to help elicit the swallowing, gagging, and vomiting reflexes.14 Damage to these areas can often result in nausea and emesis as seen in the patient presented here.16
Cranial nerve X also has widespread connections to the head, neck, and thorax.14 Specifically, this nerve innervates the levator veli palatini, which is responsible for elevation of the soft palate.14 Functionality can be easily assessed by having the patient stick their tongue out and say “ahh” to evaluate for palatal weakness or paralysis of the constrictor muscles. Depending on what side is lesioned, the examiner may observe palatal droop ipsilateral to the lesion and deviation of the uvula contralateral to the lesion.14 As seen in this case, however, there was an inability of the soft palate to elevate on either side, which suggested bilateral cranial nerve X damage.
Assessing for, let alone differentiating, a cranial nerve IX from a cranial nerve X palsy is difficult as they both have similar functions and are often lesioned together based on proximity. As both function in the production of speech, it is important to ask about dysphonia and dysarthria when suspecting impairment. This may be assessed by having the patient recite a phrase for the examiner to listen for hoarseness or vocal tone abnormalities.14 Weight loss is another concerning finding as these cranial nerves make up the nucleus solitarius, which is involved in integrating the perception of satiety and taste. It is thought that compression of the nucleus solitarius, in the medulla, can lead to reduced appetite and subsequent weight loss.16 In this case, the patient’s weight loss is likely multifactorial and related to both appetite loss and dysphagia.
The nucleus solitarius also has connections to the brainstem reticular formation that is responsible for arousal as well as emesis.17 The reticular formation is a brainstem structure that is made up of a diffuse network of neurons projecting from multiple brainstem nuclei to the cortex. The vomiting center, also known as the area postrema or chemoreceptor trigger zone, is a part of the reticular formation that is located in the dorsal lateral medulla. Compression of the vomiting center also likely contributed to this patient’s symptoms of emesis.17
In contrast to the previously mentioned cranial nerves, cranial nerve XII is solely responsible for movement of the tongue during speech, food manipulation, and swallowing.15 It innervates all tongue muscles aside from the palatoglossus muscle, which is innervated by cranial nerve X.18 Dysfunction of cranial nerve XII may present as diminished mobility, fasciculations, or wasting of the tongue. This can be tested by having the patient stick their tongue straight out. If a unilateral lesion is present, the tongue will deviate toward the affected side. Fasciculations or wasting may arise in chronic lower motor neuron lesions. As seen in this case, bilateral dysfunction of the hypoglossal nerve was demonstrated by bilateral tongue fasciculations.
It is important to note that the accessory nerve (cranial nerve XI) also originates at the level of the low medulla in the nucleus ambiguous, with cranial nerves IX and X, and exits the skull via the jugular foramen, again with cranial nerves IX and X. Cranial nerve XI is responsible for innervation of the sternocleidomastoid and trapezius muscles.15 The functionality of this nerve can be tested by having the patient shrug their shoulders against force or by having them turn their head against resistance.15 Dysfunction is often displayed as fasciculations or wasting. Given that these three cranial nerves run so closely together, it is likely that there may have been some apparent damage to this nerve as well. However, no definite dysfunction was noted during the examination.
Recall, the patient also demonstrated ataxia suggestive of cerebellar damage. The cerebellum is known to regulate posture, balance, coordination, and eye movements. It resides in the posterior cranial fossa and is separated from the brainstem by the fourth ventricle. The cerebellar flocculonodular lobe is generally responsible for equilibrium, ocular movements, and vestibulo-ocular reflexes. The anterior lobe of the cerebellum regulates tone and posture, whereas the posterior lobe controls voluntary movements. The central vermis is responsible for receiving auditory, visual, and vestibular input as well as sensorimotor input from the head, trunk, and proximal limbs.19
Those with dysfunction of the cerebellum often present with impaired gait and ataxia but could also demonstrate abnormal coordination of limbs, dysmetria (inability to control distance/speed/motion of movement), and nystagmus.19 Difficulty with balance is often seen in early stages of cerebellar disorders, and difficulty with walking is seen later in the disease state.20 Assessment of cerebellar function often starts with testing for ataxic gait, which often has a “drunken appearance” and may be characterized by widened stance, unsteadiness, lateral veering, and clumsiness with poor coordination of the legs and feet.19 There may also be reduced walking speed, cadence, step stride length, and swing phase with an increase in base width, stride time, step time, stance phase, and double limb support. Tandem gait, having the patient walk heel-to-toe in a straight line, is another common assessment. Those with cerebellar ataxia would find this difficult.19
Romberg testing is another easy way to assess for cerebellar dysfunction. During this test, the patient is to stand, with closed eyes, and maintain their balance without oscillating or falling. Those with cerebellar damage may have difficulty maintaining their balance in this way, demonstrating postural body tremor.21 Asynergia, the inability to create fluid motion, can also be evaluated with finger-to-nose testing. Patients with cerebellar dysfunction may demonstrate dysmetria, overshoot of a target, or may present with “intention” or “kinetic” tremor.19 Kinetic tremor is an oscillatory movement that will progressively increase in amplitude during the terminal phase of voluntary movement and will disappear with rest.21 Dysdiadochokinesis, impaired rapid alternating movement, may also be seen.20 From an ocular perspective, those with cerebellar dysfunction may also present with downbeat nystagmus, gaze-evoked nystagmus, and periodic alternating nystagmus. Patients may also exhibit square wave jerks, microsaccades, saccadic intrusions, and slowed saccades.20
Conclusion
Central nervous system hemangioblastomas, although benign and nonmetastasizing, can cause significant neurologic disability due to mass effect on the brainstem and cerebellum as well as blockage of cerebrospinal fluid and subsequent increased intracranial pressure. As seen in this case, mass compression of critical brain structures can cause an array of symptoms that may delay accurate diagnosis. The ability to recognize papilledema allows eyecare providers to be the first member of the health care team to relate a patient’s symptoms to a more ominous process. This, along with thorough history-taking and cursory neurologic examination, is key to understanding the clinical presentation, determining specific anatomic localization, and recognizing the need for emergent workup and treatment.
No identifiable health information was included in this case report.
Take Home Points
-
An intracranial mass, depending on the location, can cause varied systemic symptoms that can delay an accurate diagnosis.
-
Thorough history taking and detailed clinical examination, including cursory neurologic examination, are key to understanding and localizing a pathologic presentation.
-
It is important that eye care providers understand the eye-brain connection and be able to perform a cursory neurologic examination to allow for a quicker diagnosis and potential treatment.
Conflicts of Interest
The authors declare no conflicts of interest.