INTRODUCTION
Interstitial keratitis is a stromal immune inflammation due to herpes simplex virus, herpes zoster ophthalmicus (hzo), syphilis, tuberculosis, or Cogan syndrome.
Refractile deposits in the cornea are rare, and they can be caused by infection.1 The most common organisms are Streptococcus Viridans, Mycobacteria sp. and Candida sp. Corneal collagen cross-linking,2 scleral contact lens3 wear, biologic agents such as Secukinumab,4 systemic conditions (myeloma, monoclonal gammopathy, gout, cystinosis, abnormalities of lipid deposition), and dystrophies (Schnyder or Bietti) have also been associated with refractile deposits in the cornea.
Post-LASIK ectasia,3 corneal collagen cross-linking,2 and scleral lens wear3 have all been associated with refractile deposits in the cornea. Mycobacterium chelonae infection has been reported many years after LASIK.5 We are aware of only 1 reported case in a patient wearing scleral contact lenses for post-LASIK ectasia.3 In their case, no corneal scrape or biopsy was performed to investigate for infection. The condition has also been reported in the weeks following corneal collagen cross-linking,2 a widely accepted treatment for keratectasia.
Omalizumab, an anti-immunoglobulin E monoclonal antibody administered by subcutaneous injection used to treat asthma and chronic spontaneous urticaria, is another potential contributor. This medication binds immunoglobulin E, preventing attachment to inflammatory mediator cells such as mast cells, thereby suppressing the immunoglobulin E–mediated allergic response.
Monoclonal gammopathies are a range of conditions in which plasma cells produce abnormal monoclonal antibodies that can be deposited in the cornea. These gammopathies include silent monoclonal gammopathy of undetermined significance, multiple myeloma, and Waldenstrom macroglobulinemia.
The unique features of this case of interstitial keratitis are the absence of a clear infective cause and the long duration since LASIK and cross-linking procedures. Current scleral contact lens wear and systemic anti-immunoglobulin E therapy may also have played a role in the etiology of this vision-threatening condition. No identifiable health information was included in this case report.
CASE REPORT
The patient underwent bilateral LASIK in 2001 at age 27 years for 7 dioptres of myopia. There were no clinical or topographic findings of ectasia. She had worn daily-wear soft contact lenses full-time for the preceding 10 years.
In 2006, bilateral post-LASIK ectasia was diagnosed and after significant progression of this condition over the following few years, cross-linking was performed on both eyes in 2009. For cross-linking treatment, the cornea was soaked with 20% ethanol for 20 seconds and then the epithelium manually debrided. Normotonic riboflavin was then instilled for 15 minutes, followed by ultraviolet light for 30 minutes. Hypotonic riboflavin was instilled for the last 5 minutes of the ultraviolet phase to thicken the stroma because the central corneal pachymetry fell below 400 µm. The postoperative period was notable for marked pain for the first day but was otherwise unremarkable.
For the right eye, the soaking phase required hypotonic riboflavin for 1 hour because of reduced corneal pachymetry and the postoperative phase complicated by development of a small central dendritic ulcer and mild anterior stromal haze. An epithelial swab for herpes simplex virus was negative by polymerase chain reaction, with the haze resolving over a few months with fluorometholone acetate 0.1% drops.
Following diagnosis of post-LASIK keratectasia in 2006, 3 different types of contact lenses were worn. Initially, rigid corneal contact lenses were prescribed; however, they could not be tolerated because of lens instability and discomfort. Hybrid contact lenses (SynergEyes), comprising a central-zone rigid lens circumferentially bonded to a peripheral-zone soft skirt, were then worn. These provided visual acuities of right and left 6/6. Because of progression of the keratectasia, she was then refitted with scleral lenses, which provided visual acuities of right and left 6/6, as well as uneventful wear for the next 11 years. The lenses were inserted with Lens Plus saline solution preserved with 0.005% OcuPure, which decomposes when exposed to light, thus substantially reducing the risk of a toxic/allergic reaction. Cleaning was performed by digital rubbing with an alcohol-based cleaner (Oté Clean) followed by rinsing with saline, then contact lens disinfection was achieved by storing the lenses overnight in hydrogen peroxide solution (AOSept Plus).
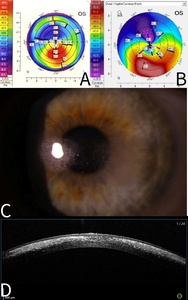
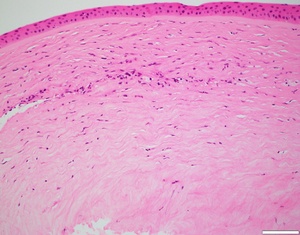
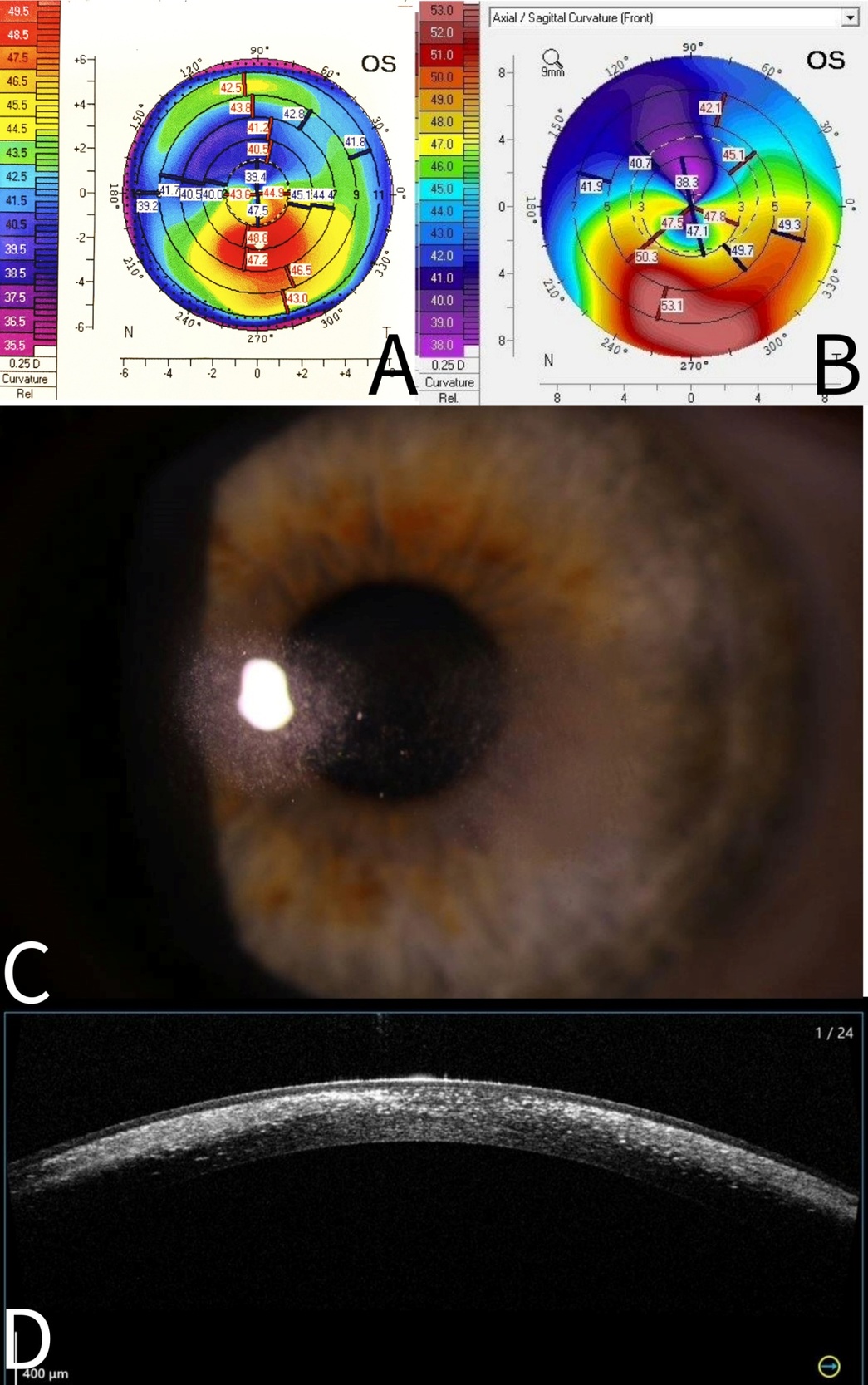
The patient presented in 2022 with painless multiple mid-stromal fine refractile deposits in the nasal and temporal paracentral left cornea without stromal inflammation, epithelial defect, or anterior chamber reaction (Figure 1). Examination of the lens fit revealed that it was still well fitting, exhibiting complete corneal clearance. The remainder of the left and right eye examinations were unremarkable. Anterior segment optical coherence tomography demonstrated refractile deposits predominantly posterior to but not tracking within the LASIK flap interface. There was no clinical epithelial in growth. Typical topographic ectasia findings of corneal thinning with inferior steepening were present bilaterally (Figure 1). There were no asymmetric topographic findings that might explain a predisposition on the left to refractile deposits forming. In vivo confocal microscopy demonstrated multiple hyperreflective needle-like deposits in the anterior stroma (Figure 2). Initial corneal biopsy performed by 200-µm depth lamella dissection, sent for microbiological and histological assessment and repeated, were both unremarkable. The excised penetrating corneal donor button histology demonstrated stromal lymphocytes, scattered foamy macrophages, and an increase in density and reactivity of keratocytes; however, there were no crystals (Figure 3). No stromal vascularization or viral inclusions were seen. Immunohistochemistry confirmed the presence of macrophages (CD163+) and T cells (CD3+, CD4+). Immunohistochemistry was negative for CMV, herpes simplex virus 1, and herpes simplex virus 2.
The patient’s systemic history consisted of mild allergic rhinitis; Venlafaxine, a serotonin selective reuptake inhibitor for perimenopausal flushing; Omalizumab for chronic spontaneous urticaria; Rizatriptan for migraines; oral Ibuprofen or Naproxen for musculoskeletal pain, and Liraglutide for weight loss and HRT.
Topical corticosteroids had no effect, neither improving nor worsening the condition. In addition to routine microscopy and culture, the following stains were all negative: auramine-rhodamine and Ziehl-Neelsen for acid-fast bacilli such as Mycobacterium, Periodic Acid-Schiff, and Silver Methanamine for fungi. Cultures and polymerase chain reaction for Microsporidia and culture for Mycobacterium and were negative.
The patient had no significant systemic symptoms, full blood count and erythrocyte sedimentation rate/C-reactive protein investigations repeated over 2 to 3 years were normal. Blood serum testing was unremarkable, there were no paraprotein bands and lipid, calcium, and uric acid profiles normal. Serology for tuberculosis and syphilis was negative. There were no urine Bence Jones proteins.
DISCUSSION
There are several potential etiologies in this case of interstitial keratitis. We found no infectious cause, as test results for virus, fungi, nontuberculous mycobacteria, and Microsporidia were all negative. Microsporidia were tested for because although rare, they have a wide range of presentations. There was no history of long-term topical steroid use or evidence of a systemic cause. Past LASIK, cross-linking, current scleral lens wear, and current systemic anti-immunoglobulin E therapy may all have played a role.
We are aware of one similar case report of bilateral arcuate paracentral refractile deposits in a patient wearing scleral contact lenses many years after post-LASIK keratectasia.3 This case was similar to ours in that the refractile deposits were arcuate and paracentral and optical coherence tomography confirmed the changes were deep to the LASIK flap interface. In both cases, the scleral contact lens demonstrated complete corneal clearance; however, our case differed in that it was post–cross-linking and unilateral (with the other eye exhibiting no such signs). The authors hypothesize that although the scleral contact lens fit was good, hydrostatic pressure exerted by the scleral contact lens during wear and removal on the tear film, combined with a compromised ectatic cornea, might have led to the tears film’s salt crystals precipitating in the stroma as a biomechanical effect. Our patient would remove the scleral lens without the recommended loosening of the seal first, thereby increasing hydrostatic pressure on the cornea. The hyperreflective needle-like opacities on vivo confocal microscopy findings in our case have previously been reported.6
We postulate that a contributing factor was scleral contact lens–induced chronic low-grade stromal inflammation causing keratocyte activation7 and lysis of the extracellular matrix,8 facilitating crystal spread between the corneal lamellae. In support, studies on keratoconus eyes have demonstrated increased tear inflammatory mediators that cause degradation of the corneal stroma. Lema et al9 demonstrated in patients with keratoconus, many who wore hard contact lenses, increased concentrations of pro-inflammatory cytokines: interleukin 6, tumour necrosis factor alpha, and matrix metalloproteinase 9 compared with controls. Yeung et al10 demonstrated that with scleral contact lens wear, levels of interleukin 1β, tumour necrosis factor alpha, and matrix metalloproteinase 1 all increased as limbal clearance increased; however, they found no difference in matrix metalloproteinase 9. Matrix metalloproteinases cause degradation of the corneal stromal extracellular matrix, classified by their substrate: matrix metalloproteinase 1 acts on the commonest corneal collagen; type 1 and matrix metalloproteinase 9 acts on gelatin components of the extracellular matrix. Keratocyte have been shown to become activated in response to inflammation, differentiating their phenotype towards corneal fibroblasts. It has been hypothesized that this effect is mediated by interleukin 1β in an interplay with transforming growth factor beta.7 A reduction in goblet cell density and squamous cell metaplasia has also been demonstrated in long- term scleral lens wear,11 which might have played a role.
Although scleral contact lenses can cause hypoxia and increase corneal inflammation, as evidenced by higher limbal clearance causing an increase in tear inflammatory mediators,10the authors don’t believe that significant clinical hypoxia played a role in this case. Our patient’s scleral lens central thickness was 300 µm in the Acuity 200 material, which has a very high oxygen permeability of 200 Barrer. Her lenses always demonstrated complete corneal clearance, less than 200 µm centrally and less than 60 µm at the limbus. Given the combination of this lens-corneal relationship and high oxygen-permeability lens material, the oxygen transmissibility of the patient’s lenses always satisfied the theoretical recommendations for minimizing corneal hypoxia, as recommended by Michaud et al.12 Further, no signs of corneal hypoxia were observed at any aftercare appointment.
Omalizumab is a potential cause, used to treat chronic spontaneous urticaria in this patient. This immunoglobulin G monoclonal antibody binds immunoglobulin E, preventing binding of eosinophils, mast cells, and basophils, thereby suppressing the allergic cascade. There is one reported case of a biologic agent, Secukinumab, causing a similar refractile-type appearance in the cornea.4 These medications have different targets; however, Omalizumab inhibits immunoglobulin E and Secukinumab inhibits interleukin 17A.
There was no evidence on symptoms or testing of a systemic cause for interstitial keratitis or conditions associated with refractile particle deposition. There was no clinical history or investigation findings suggestive of herpes zoster ophthalmicus, syphilis, or tuberculosis. Blood and urine test results for conditions associated with abnormal protein, calcium, lipid, and uric acid deposition were all unremarkable. Full blood count and erythrocyte sedimentation rate/C-reactive protein levels, repeated over 2 years were all normal, making a systemic amyloid deposition process is unlikely.
CONCLUSION
In summary, this case demonstrates the importance of considering scleral lens wear in the etiology of unique presentation of interstitial keratitis, particularly in cases with a history of LASIK, cross-linking, or biologic use.
TAKE HOME POINTS
- Scleral lens wear, corneal collagen cross-linking, and past LASIK should all be considered possible etiological factors in interstitial keratitis.
- Omalizumab, a monoclonal antibody against immunoglobulin E, a new class of anti-allergy medication, may also play a role.
- Refractile deposits can occur in scleral contact lens wear, even with high oxygen transmission material, no clinical hypoxia. and good fit.
No identifiable health information was included in this case report
CONFLICTS OF INTEREST
The authors declare no conflicts of interest
FUNDING
The authors declare no funding sources
ACKNOWLEDGEMENT
The authors would like to thank Professor John Dart for his guidance.