INTRODUCTION
Multiple myeloma is an incurable hematologic cancer characterized by the presence of malignant plasma cells accumulating in the bone marrow.1 It is commonly diagnosed between the sixth and seventh decade of life with median age at diagnosis of 69 years.2 Patients may develop relapsed or refractory multiple myeloma when their disease is nonresponsive to the chosen line of therapy in patients who had achieved a minimal response or better at some point previously in the treatment of their disease.3 Common treatments include autologous hematopoietic stem cell transplantation, proteasome inhibitors, immunomodulatory agents, anti-CD38 monoclonal antibodies, and corticosteroids.1,4 Patients who develop relapsed or refractory multiple myeloma have a particularly poor prognosis with a median overall survival of 9.3 months compared with 126 months in less aggressive forms of the disease.1,5
Belantamab mafodotin (belamaf; BLENREP, GlaxoSmithKline), a first-in-class antibody-drug conjugate, was approved by the US Food and Drug Administration in 2020 for the treatment of adult patients with relapsed or refractory multiple myeloma who had received at least 4 other therapies including an anti-CD38 monoclonal antibody, a proteosome inhibitor, and an immunomodulatory agent.6 It is composed of an afucosylated, humanized immunoglobulin G1 anti-B-cell maturation antigen monoclonal antibody conjugated to a microtubule disrupting agent monomethyl auristatin F.7,8 It targets B-cell maturation antigen, which is a cell-surface receptor expressed on normal mature B-cells and multiple myeloma cells.7,8 Once bound to the B-cell maturation antigen receptor, it kills the multiple myeloma cell via a multimodal mechanism, involving the delivery of monomethyl auristatin F inducing apoptosis.7,8 The most common adverse event with this medication is corneal epitheliopathy resembling microcystic edema, which has been named corneal pseudomicrocysts.8,9 These corneal pseudomicrocysts occurred in 72% of patients in the DREAMM-2 study.8,9 Initially, the corneal changes appear near the limbus and extend to the central cornea as treatment duration increases causing blurry vision and dry eye.8,9 Corneal pathology is reversible upon discontinuation of the medication.8,9 At the time of data cutoff for DREAMM-2, 77% of patients recovered from their first occurrence of grade 2 or higher corneal pseudomicrocysts with a median time to resolution of 86.5 days.9 No identifiable health information was included in this case report.
CASE REPORT
A 53-year-old African American woman presented for an eye examination prior to initiating belantamab mafodotin therapy to treat relapsed and refractory multiple myeloma. Entering best-corrected visual acuities were 20/20 in both right and left eyes at distance and near through her habitual spectacles. Lensometry results were +3.50 - 0.75 × 175 ADD +2.00 in the right eye and +3.75 - 1.00 × 175 ADD +2.00 in the left eye. Manifest refraction yielded no change to habitual spectacles. Her anterior and posterior segment examination findings were unremarkable. Specifically, there were no abnormal findings of the cornea in either eye. Intraocular pressures were 16 mm Hg in the right eye and 15 mm Hg in the left eye measured by Goldmann applanation. The patient was scheduled for a 3-week follow-up to monitor for corneal toxicity per treatment protocol.
Follow-Up 1
The patient was seen for follow-up 27 days after the first infusion of belantamab mafodotin. At this time, the patient denied blurry vision or symptoms of ocular dryness. Entering best-corrected visual acuities were 20/20 in both right and left eyes. Anterior segment slit lamp examination with sodium fluorescein was unremarkable in both eyes, and the patient’s corneas were still clear with no signs of corneal staining or corneal pseudomicrocysts at this time. The Risk Evaluation and Mitigation Strategy Eye Care Professional Consult Form was filled out and sent to her oncologist. A follow-up examination was scheduled for 3 weeks later to monitor for corneal toxicity prior to the next infusion.
Follow-Up 2 (Two Infusions Received, 50 Days After Initial Belantamab Mafodotin Infusion)
The second infusion of belantamab mafodotin was administered 23 days prior to her second follow-up visit. Once again, the patient denied blurred vision or symptoms of ocular dryness and best-corrected visual acuities were 20/20 in both right and left eyes. Slit lamp examination revealed mild, nonconfluent corneal pseudomicrocysts in the peripheral corneas of both eyes. No corneal staining was noted with sodium fluorescein on either eye. Intraocular pressures were 16 mm Hg in the right eye and 14 mm Hg in the left eye by Goldmann applanation. These corneal findings were consistent with grade 1 on the keratopathy and visual acuity scale (Table 1). Based on examination findings, it was recommended to instill preservative-free artificial tears 4 times per day into both eyes. The Risk Evaluation and Mitigation Strategy Eye Care Professional Consult Form was filled out and sent to her oncologist. The patient was scheduled for follow-up evaluation approximately 3 weeks later.
Follow-Up 3 (71 Days After Initial Belantamab Mafodotin Infusion)
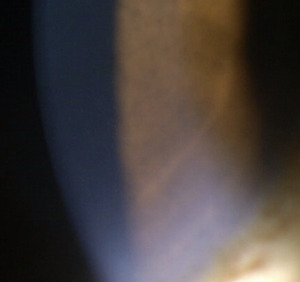
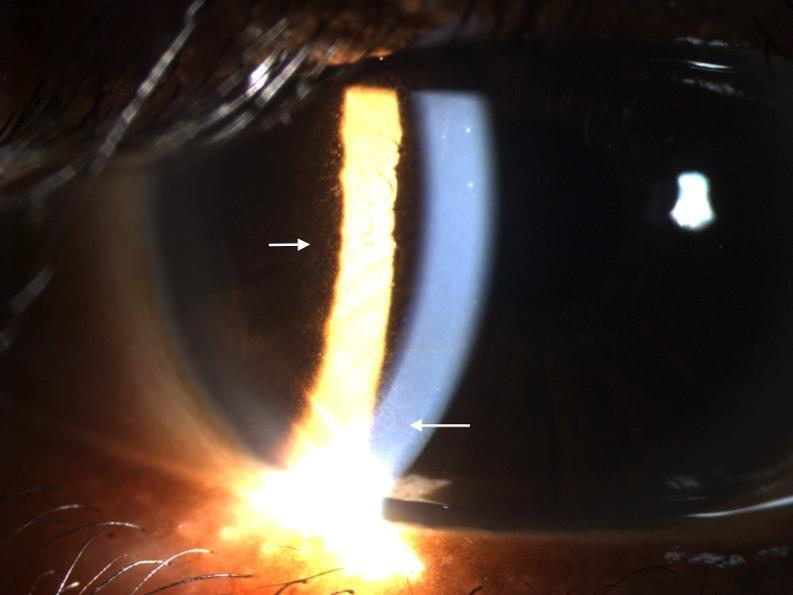
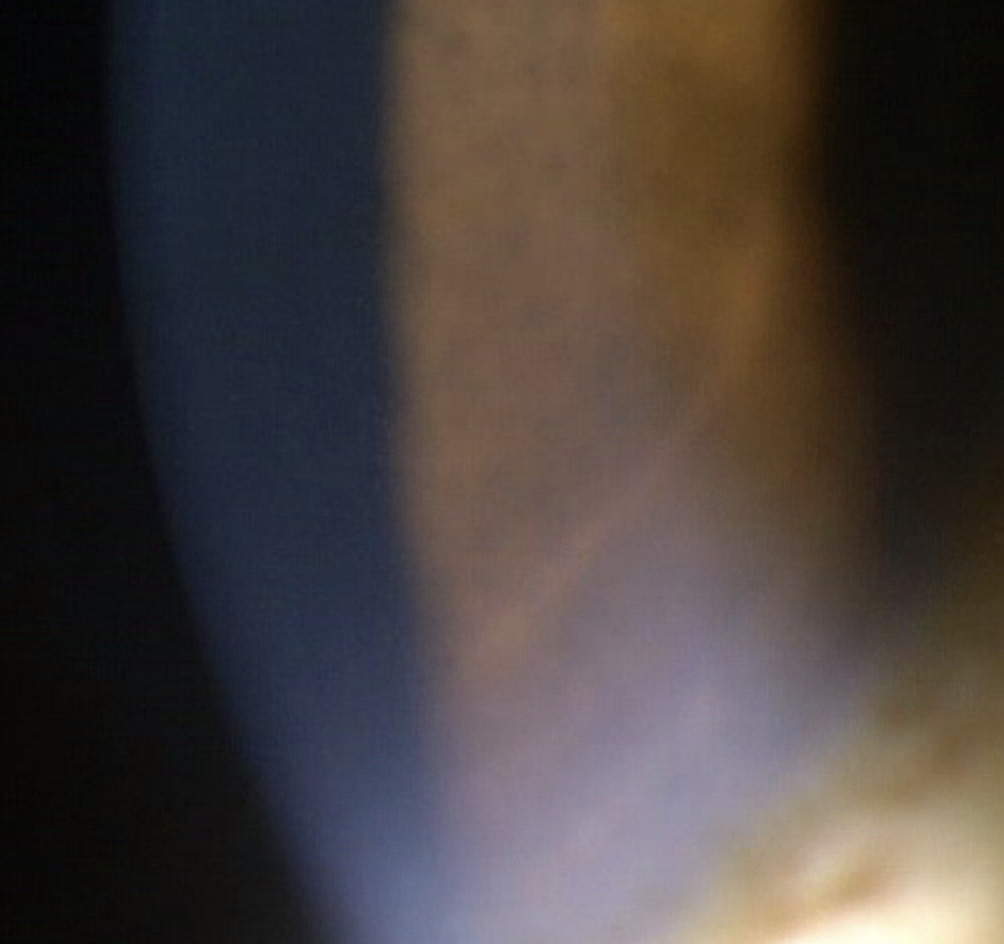
The third infusion of belantamab mafodotin was administered 21 days prior to her third follow-up visit. The patient denied any changes in vision but did note increased tearing in both eyes compared with the previous examination. The patient was not using preservative-free artificial tears four times a day as prescribed. Best-corrected visual acuities were 20/20 in the right eye and 20/25 in the left eye. Slit lamp examination revealed peripheral and paracentral confluent corneal pseudomicrocysts in both the right and left eye (Figures 1 and 2). No corneal staining was noted with sodium fluorescein. Her tear break-up time was 10 s in the right eye and 11 s in the left eye. Both puncta were patent with a positive Jones I test result. Intraocular pressures were 15 mm Hg in the right eye and 14 mm Hg in the left eye by Goldmann applanation. She deferred dilation, but an undilated fundus examination was performed and was unremarkable. These corneal findings were consistent with grade 2 on the keratopathy and visual acuity scale.8 Based on her complaint of epiphora, she was instructed to use preservative-free artificial tears 4 times per day in both eyes. The Risk Evaluation and Mitigation Strategy Eye Care Professional Consult Form was filled out and sent to her oncologist. The patient was scheduled for a follow-up examination 3 weeks later.
Follow-Up 4 (124 Days After Initial Belantamab Mafodotin Infusion / 53 Days After Grade 2 Findings)
The patient returned 53 days later. Her fourth infusion had been delayed due to corneal findings noted at her previous examination; her oncologist requested repeat ocular evaluation prior to resuming therapy. The patient reported increasingly blurred vision in both eyes as well as consistent tearing, but she reported that she had not used artificial tears as recommended at her last examination. Entering best-corrected visual acuities were 20/25 in both right and left eyes. Slit lamp examination revealed confluent corneal pseudomicrocysts in the peripheral and paracentral corneas of both eyes. No corneal staining was noted with sodium fluorescein. Intraocular pressures were 15 mm Hg in the right eye and 15 mm Hg in the left eye by Goldmann applanation. Based on the keratopathy and visual acuity scale (Table 1), findings were classified as grade 2 corneal pathology. Once again, she was instructed to use preservative-free artificial tears 4 times daily to stabilize the tear film. The Risk Evaluation and Mitigation Strategy Eye Care Professional Consult Form was filled out and sent to her oncologist. The patient was scheduled for a follow-up examination 3 weeks later.
Follow-Up 5 (182 Days After Initial Belantamab Mafodotin Infusion / 111 Days After Grade 2 Findings)
The patient received her fourth infusion the same day as her fourth follow-up visit. The dosage was reduced due to corneal findings. She reported an improvement in vision and decreased tearing compared with her previous appointment. Entering best-corrected visual acuities were 20/20 in both right and left eyes. Slit lamp examination was unremarkable; no corneal pseudomicrocysts were noted in either eye (Figure 3). Intraocular pressures were 16 mm Hg in the right eye and 14 mm Hg in the left eye by Goldmann applanation. The Risk Evaluation and Mitigation Strategy Eye Care Professional Consult Form was filled out and sent to her oncologist. The patient was schedule for a follow-up examination 3 weeks later.
She was lost to follow-up at this point for many months. When she returned, she had discontinued belantamab mafodotin treatment and there were still no corneal findings present.
DISCUSSION
Multiple myeloma is a hematologic malignancy characterized by the presence of malignant plasma cells in the bone marrow.1 Accumulation of these malignant cells can cause destructive bone lesions, acute kidney injury, anemia, and hypercalcemia.1 The exact cause of the disease is still unclear, but some risk factors have been identified. Those risk factors include male sex, occupations as a firefighter, obesity, Agent Orange exposure, and 9/11 first responders.1,10–13 There are various treatment options available for patients with multiple myeloma including proteasome inhibitors, immunomodulatory agents, anti-CD38 monoclonal antibodies, corticosteroids, and autologous hematopoietic stem cell transplantation.1,14 The overall goal with treatment is to reduce the amount of malignant plasma cells in the bone marrow.1 When treatment is started, most patients have a positive response to the initial treatment.14 However, some patients do not respond to initial treatment and are classified as having primary refractory disease, accounting for 6.7% of those with multiple myeloma.14 Eventually, the disease will relapse, creating a complex and challenging scenario.14,15 Even with a combination of the above treatments, relapsed or refractory multiple myeloma is difficult to manage and has a poor prognosis with a 5-year survival rate of 55%.6,14 Belantamab mafodotin was granted accelerated approval by the US Food and Drug Administration in August 2020 following the results of DREAMM-2 (Driving Excellence in Approaches to Multiple Myeloma), which had an overall response rate of 31%, and 73% of patients had a duration of response of 6 months.6,8 Patients receive 2.5 mg/kg by intravenous infusion every 3 weeks.6,8 Belantamab mafodotin is composed of an afucosylated, humanized immunoglobulin G1 anti-B-cell maturation antigen monoclonal antibody conjugated to a microtubule disrupting agent monomethyl auristatin F.7,8 It works by binding to B-cell maturation antigen and inducing apoptosis through various mechanisms.7,8 B-cell maturation antigen is a receptor expressed on all malignant plasma cells and is essential for their survival.7,8
The most common adverse event in DREAMM-2 was corneal pseudomicrocysts previously referred to as microcystic-like epithelial changes, which occurred in 72% of patients receiving belantamab mafodotin.8,9 The physiological mechanisms by which the medication causes corneal epithelial changes are not completely understood; however, it is theorized that the medication enters the cornea either through the limbal vasculature or the tear film becoming internalized by the corneal epithelium in the basal layers via macropinocytosis.9 Once the medication is internalized by the epithelium, it induces apoptosis of the corneal epithelial cell.9 The affected epithelial cell continues along its normal path, migrating centrally and anteriorly in the cornea.9 These migrating apoptotic cells are visualized as hyperreflective opacities.9 As they continue to migrate toward the visual axis, visual acuity may be reduced. Eventually, the affected cells migrate to the surface of the cornea and are sloughed off. Over time, new epithelial cells unaffected by belantamab mafodotin replace apoptotic cells, and vision returns to baseline.9
Farooq et al reviewed corneal examinations from DREAMM-2 and found the median time to onset of corneal pseudomicrocysts was 37 days.9 They were first observed following the first dose in 25% of patients.9 With each progressive dose, the incidence of corneal pseudomicrocysts increases; 69% of patients experience their first corneal pseudomicrocysts event by the fourth dose.9 The patient described in this case report first developed corneal pseudomicrocysts in the peripheral cornea prior to her third dose, approximately 50 days after starting the medication, but this did not initially affect visual acuity. Recommendations for management stipulate that additional doses of belantamab mafodotin should be delayed or reduced if grade 2 corneal findings are present, as was the case for the presenting patient.9 The patient’s fourth infusion of belantamab mafodotin was initially delayed by her oncologist pending further ocular evaluation. When ocular findings remained consistent, per manufacturers’ protocol and oncologists’ discretion, the dosage of her fourth and final infusion of belantamab mafodotin was reduced.
Fortunately, 77% of patients fully recovered from grade 2 or greater corneal findings within a median of 86.5 days.9 Once again, this patient’s course of recovery was similar to previous reports. Her corneal findings had resolved within 111 days following the dose modification. DREAMM-2 looked at prophylactic treatments with topical corticosteroid eye drops to mitigate ocular adverse events. They found that topical corticosteroid eye drops were ineffective at mitigating ocular adverse events including corneal pseudomicrocysts.9 Therefore, topical corticosteroids were not prescribed for the patient evaluated in this case report.
Recently, belantamab mafodotin was removed from the market and the US Food and Drug Administration withdrew the US license to manufacture the medication in February 2023. DREAMM-3 failed to meet its primary endpoint and failed to meet the requirements of the US Food and Drug Administration’s Accelerated Approval regulation.16 However, more recent studies (DREAMM-7 and DREAMM-8) have shown increased progression-free survival when belantamab mafodotin is used in combination with bortezomib (Velcade, Millennium Pharmaceuticals, Inc) or pomalidomide (Pomalyst, Celgene) and dexamethasone.17,18 There was a higher incidence of ocular adverse events in these trials with the addition of belantamab mafodotin instead of the current gold standard of daratumumab (Darzalex, Janssen Biotech, Inc), an anti-CD38 monoclonal antibody.17,18 Patient-reported outcomes for quality of life found that fatigue and decreased appetite were the adverse events that impacted health. Blurred vision did not have a substantial negative impact.19
Although the drug used by the patient described here is no longer on the market, similar corneal findings have been observed in other drugs of the same class.20 Antibody-drug conjugates are a promising innovation in cancer treatments. They are designed to selectively target and deliver their cytotoxic payload to their target cancer cell.21 Of the 11 US Food and Drug Administration approved antibody-drug conjugates, corneal pseudomicrocysts have been observed in 3 of them.20 Antibody-drug conjugates that use monomethyl auristatin F as their cytotoxic payload have shown these same corneal findings.9
CONCLUSION
Antibody-drug conjugates will likely continue to be developed and used in the treatment of certain types of cancer. Eye care providers should be able to identify corneal pathology associated with the use of these medications and should work closely with oncologists to ensure appropriate treatment for patients undergoing treatment with these pharmaceutical agents.
-
No identifiable health information was included in this case report
-
The author declares no conflicts of interest
-
The author declares no funding sources
TAKE HOME POINTS
-
Antibody-drug conjugates are becoming more prevalent and cause corneal changes.
-
Corneal pseudomicrocysts are thought to be a result of an off-target mechanism; corneal epithelial abnormalities resolve after dose delay or dose modification.
-
Communication and cooperation with oncologists in the management of patients on these medications will optimize treatment effects while minimizing ocular toxicity.
ACKNOWLEDGMENTS
Thanks to Dr Muriel Schornack and Dr Jennifer Harthan.