INTRODUCTION
The development and use of scleral lenses has surged due to today’s improved designs and options. Scleral lenses, initially meant as visual correction for severely irregular corneas, have now evolved to a more diverse spectrum of indications due to their increased comfort and ability to vault steeper eyes.1 They are now used for ocular surface protection and rehabilitation, such as in cases of severe dry eye and incomplete lid closure. The scleral lens fluid reservoir promotes corneal healing while protecting the ocular surface from the lids and environment.2 In many cases, scleral lenses have been reported to postpone or even prevent surgical intervention in ocular surface disease.1 Although scleral lenses are widely used in adults, their application in the pediatric population is documented in only a limited number of case reports.3–6
The case presented here discusses a young toddler with exposure and neurotrophic keratopathy secondary to a posterior fossa tumor and its surgical removal. Her ocular surface was successfully rehabilitated with a therapeutic scleral lens. No identifiable health information was included in this case report.
CASE REPORT
Initial Visit
A 20-month-old girl was referred for a medical contact lens fitting. She has a history of exposure and neurotrophic keratopathy of her left eye, leading to frequent recurrent corneal erosions and ulcerations. Her mother had been applying bland ointment every 2 hours, erythromycin ointment 3 times daily, and a tape patch at night over the left eye. She was currently comanaged by a corneal specialist and a pediatric ophthalmologist within the eye institute who, at this time, recommended a tarsorrhaphy for protection. However, given the patient’s extensive surgical history, the patient’s mother sought other options to avoid further surgical intervention.
The patient’s medical history was significant for a large posterior fossa hemorrhagic mass at age 15 months, with a recurrence of the tumor 4 months after the initial diagnosis. She underwent 2 craniotomies, 2 periods of chemotherapy with stem cell infusion, and 1 episode of radiation. The patient had multiple ocular manifestations secondary to the brain tumor and its surgical removal, including a left cranial nerve IV, V, VI, and VII palsy. She had a left 60-65 prism diopter esotropia, resulting in deep strabismic amblyopia.
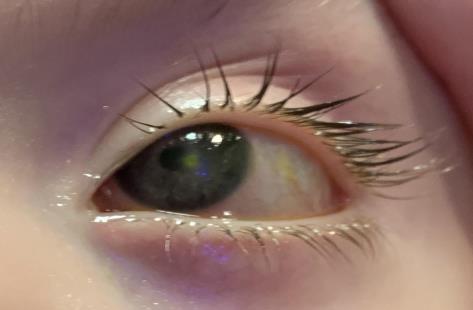
The patient’s uncorrected entering acuity was central, steady, and maintained in the right eye and central, steady, and unmaintained in the left eye, indicating that the patient was not able to hold fixation on the target. Handheld slit lamp examination revealed a 2x2 mm round paracentral epithelial defect with anterior stromal edema on the left cornea (see Figure 1 and 2). The anterior segment of both eyes was otherwise unremarkable. The posterior segment examination of both eyes, completed 4 months prior by her pediatric ophthalmologist, was unremarkable. The patient’s neurotrophic keratopathy in the left cornea was classified as stage 2 according to the Mackie classification7 and stage 4 based on the Neurotrophic Keratitis Study Group classification.8
A scleral contact lens was fit on the patient’s left eye to protect her ocular surface from exposure and prevent future epithelial defects. A smaller scleral lens of 14.8 diameter was selected to help with easier application and removal. After a diagnostic lens fitting was performed using a handheld slit lamp, an initial lens was determined. Our goal was to achieve a lens with a central fluid reservoir thickness of approximately 350 microns after application.1 After completing a retinoscopy over the lens, a prescription was determined. The parameters of the lens ordered was Custom Stable Elite / Base Curve 8.04 / Diameter 16.80 / Power +1.00.
At this visit, the patient’s mother demonstrated successful lens application and removal of the scleral lens. To prevent bubbles during application, she was instructed to fill the lens with a mixture of nonpreserved saline solution and nonpreserved Refresh Celluvisc solution. The patient’s mother was instructed to clean the lens every night with Menicon Unique pH multipurpose solution. Erythromycin ointment was to be continued at bedtime when the lens was removed. Any residual ointment was rinsed off with saline prior to lens wear the following morning. The patient was instructed to return in 2 weeks wearing the new lens in the left eye for at least 4 hours.
Follow-Up Visit
At the follow-up, her mother reported that the patient was able to successfully wear the lens in the left eye for 6 to 7 hours a day, and her left eye appeared less red with the lens on. She also mentioned several instances in which the lens would dislocate when the patient would rub her eye.
The patient’s entering acuity was central, steady, and maintained in both eyes. To assess the scleral lens in the left eye, a Burton ultraviolet lamp was used because the patient was unable to remain steady behind a handheld slit lamp. Sodium fluorescein was applied on the eye and pushed underneath the lens using the lower lid margin. The lens appeared to be decentered inferiorly with a central 1-mm area of absent sodium fluorescein, suggesting a small area of lens touch due to insufficient sagittal depth. The inferior edge of the lens was also slightly flat. Despite the mild corneal touch of the scleral lens, the corneal defect had improved to approximately 0.50 mm round in size, and the edema resolved. Modifications were made to the lens to increase the sagittal depth by approximately 200 microns, and the diameter was increased to 16.8 mm to allow the edge to be tucked under the bottom lid to reduce lens ejection.
Follow-Up Visit
After 6 weeks of lens wear, the patient’s mother indicated that the new larger diameter scleral lens had better success staying on the eye compared with the previous lens, and she had not noted any redness or irritation with the lens on. The average lens wear time was now up to 10 hours a day.
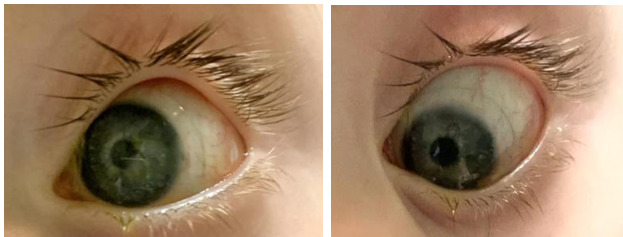
Vision was stable in the left eye and the scleral lens appeared to have adequate central and limbal clearance. The lens edges were well aligned to the sclera with no blanching or impingement of blood vessels (see Figure 3 and 4). Retinoscopy over the contact lens revealed no residual refractive error. The right cornea showed no further corneal staining, indicating a resolved corneal defect. Because an ideal fit and good tolerance was achieved with the scleral lens, the contact lens prescription was finalized. After discussing with the patient’s corneal specialist and pediatric ophthalmologist, the patient’s tarsorrhaphy was cancelled. A 6-month follow-up was scheduled to reassess the scleral lens fit.
6-Month Follow-Up
The patient’s mother reported successful scleral lens wear on most days. The patient now seemed less irritated with her eye than before. Visual acuity with Allen isolated symbols was 20/100 in the right eye, and 20/600 in the left eye. Her visual acuity in the right eye was mildly decreased, likely due to impaired visual processing associated with her brain tumor. A month prior she had a cycloplegic retinoscopy performed by her pediatric ophthalmologist, which revealed +2.00 -0.50 x 180 right eye and +1.25 sphere left eye. Her cornea was intact with rare scattered punctate staining and no epithelial defects. The patient continues to use erythromycin ointment overnight when the lens is off. Her pediatric ophthalmologist had recommended strabismus surgery to reduce strabismic amblyopia, but the patient’s mother declined. The patient was scheduled for follow-up visits every 6 months to closely monitor the scleral lens and amblyopia, and her pediatric ophthalmologist planned to see her every 3 to 4 months for additional follow-up.
DISCUSSION
Central nervous system tumors are the most common solid neoplasm and second leading cause of cancer death in children, with posterior fossa tumors being the most prevalent.9 Tumor compression or surgery can affect cranial nerve structures, leading to ocular complications, including papilledema, strabismus, neurotrophic keratopathy, and decreased visual acuity.9,10 Visual abnormalities occur in 27% to 40% of children with posterior fossa tumors and can impact self-perception, development, education, driving, employment, and quality of life.11,12
The cornea functions to protect the eye’s internal structures and contribute to the refractive power to focus light properly onto the retina.13 The anatomical integrity, transparency, and function of the cornea rely on the health of corneal sensory nerves from the ophthalmic division of the trigeminal nerve (CN V). Corneal nerves trigger blinking, induce tear production, and convert thermal, mechanical, and chemical stimuli into sensations of dryness, discomfort, or pain. They also release trophic substances crucial for epithelial integrity and wound healing. Damage to the trigeminal nerve can impair corneal sensory innervation, disrupting trophic support, the lacrimal reflex, and blinking.8,14 Corneal anesthesia can lead to neurotrophic keratopathy, a vision-threatening condition.10
Neurotrophic keratopathy involves spontaneous corneal epithelial breakdown and poor healing, classified into 3 stages according to Mackie. Stage 1 features superficial punctate keratopathy without epithelial defects. Stage 2 presents with recurrent or persistent epithelial defects with “rolled” edges and corneal edema. Untreated, this may progress to stage 3, leading to corneal ulceration and possible perforation.7 The Neurotrophic Keratitis Study Group classification system is more detailed and graded on a scale from 1 to 6, encompassing a broader spectrum of disease severity, including subclinical and more advanced stages8 (see Table 1).
In a small retrospective study by Lambley et al, the most common etiology of corneal anesthesia in children was found to be posterior fossa tumors (30.8%).10 The diagnosis of neurotrophic keratitis is challenging in children given that the hallmark of the disease is corneal anesthesia, which relies on subjective patient responses. Thus, a detailed history from the patient’s caretakers is crucial, including reports of conjunctival injection or corneal opacities.14 Accompanying facial palsy increases the risk of epithelial damage due to poor tear spreading on the ocular surface. In pediatric posterior fossa tumors, the occurrence of exposure keratitis and corneal anesthesia with facial nerve palsy was very prevalent.10 The facial nerve (CN VII) regulates blinking and tear secretion. Damage to the facial nerve can cause lid retraction, lagophthalmos, and reduced tear production.15,16 The patient in this case experienced both corneal anesthesia and facial nerve palsy, resulting in lagophthalmos and neurotrophic keratopathy.
Treatment options for neurotrophic keratopathy and facial palsy depend on the stage and severity.4,14 Mild cases are treated with preservative-free eye drops, as preservatives can damage the epithelium. For delayed epithelial healing, options include bandage or scleral lenses, tarsorrhaphy, amniotic membrane transplantation, or serum eye drops.17,18 In children with neurotrophic keratopathy, surgical treatments are avoided to preserve visual acuity. Prompt treatment is essential to prevent amblyopia, especially if corneal scarring or neovascularization occurs.14 Although there is limited literature published on the use of scleral lenses in the pediatric age group, scleral lenses have been a great treatment option for children with ocular surface disorders that failed treatment with conventional therapy.4,6,14
Scleral lenses, often referred to as a “liquid corneal bandage,” offer therapeutic benefits by protecting the ocular surface from breakdown, neutralizing corneal irregularities, and promoting surface healing by shielding the compromised epithelium from mechanical blinking and environmental insults.1–3,6 Scleral lenses are also noninvasive and act as a more approachable alternative to tarsorrhaphy in many cases.3,4 This is particularly important for our younger patients, by reducing the incidence and severity of amblyopia.
Choosing scleral lens diameter depends on various factors. Larger lenses are recommended for patients with severe dry eye to increase the tear fluid reservoir, whereas smaller lenses are easier to handle and avoid issues with peripheral scleral toricity or irregularities.1 For our patient, a smaller lens was initially selected for easier application and removal, but eventually, the diameter was increased to provide more coverage of the ocular surface.
It is important to realize that scleral lenses may pose particular barriers, such as cost, duration and complexity of the fitting process, and difficulty with lens handling.6,19 Gungor et al reported that about 20% of pediatric patients encountered a challenge or complication during scleral lens fitting and training.6 Typically, a scleral lens is assessed using an optic section behind the slit lamp; however, this method may present challenges when evaluating pediatric patients who are unable to remain steady. Instead, it may be ideal to apply sodium fluorescein to the ocular surface with gentle nudging using the lower lid to promote its flow under the lens. Using a blue penlight or a Burton Lamp outside the slit lamp, the practitioner can evaluate for an even green fluorescein pattern for adequate corneal clearance, keeping in mind that a black area indicates insufficient clearance.1 Pediatric patients may also require more training or more caretakers to assist in positioning the patient properly face down for scleral lens application.
In the case that the scleral lens was not successful in healing the corneal defect, cenegermin (Oxervate; Dompé Farmaceutici, Milan, Italy) eye drops can be considered as an alternative treatment. Approved for use in children aged 2 years and older, this ophthalmic solution is the first US Food and Drug Administration–approved drug to treat neurotrophic keratitis and can be safely used involving 6 doses per day, spaced approximately 2 hours apart for a period of 8 weeks.20 Several case reports have documented the safety and efficacy of cenegermin in restoring corneal integrity in pediatric patients with neurotrophic keratitis.20–22 At this time of diagnosis, the patient was not yet 2 years old, preventing the use of this treatment.
CONCLUSION
Central nervous tumors and their treatments can alter the visual system’s neuroanatomy, leading to ocular issues like papilledema, strabismus, and reduced corneal sensation. If untreated, these complications can negatively impact a child’s development and quality of life. Prompt diagnosis and intervention are crucial in cases of neurotrophic keratopathy to prevent amblyopia and permanent damage. Unlike conventional treatments, scleral lenses offer continuous hydration and vision optimization.
This case report contributes to the limited literature that scleral lenses are an effective noninvasive treatment for neurotrophic keratitis in children. The patient described here initially presented with neurotrophic keratopathy and exposure keratopathy, unresponsive to standard treatments. After several weeks of scleral lens wear, significant improvement in corneal health and stability was observed. The lens was well tolerated by the patient and caregivers, and follow-ups confirmed its safety and long-term effectiveness for pediatric ocular surface disease. Despite potential challenges in fitting pediatric patients, with appropriate expertise, these lenses can significantly improve the quality of life for young patients at risk of amblyopia.
TAKE HOME POINTS
- Posterior fossa tumors can directly or indirectly cause damage to neuroanatomical structures leading to ophthalmic complications, including neurotrophic keratopathy.
- Neurotrophic keratopathy involves spontaneous corneal breakdown and poor healing, progressing through 3 stages: superficial punctate keratopathy (stage 1), recurrent epithelial defects with edema (stage 2), and, if untreated, corneal ulceration and possible perforation (stage 3).
- Prompt treatment of visually threatening ocular conditions is crucial to minimize the risk of amblyopia, which can significantly affect a child’s future quality of life.
- Scleral lenses protect the ocular surface, promote healing, and offer a noninvasive alternative to tarsorrhaphy, making them especially beneficial for reducing amblyopia in younger patients.