INTRODUCTION
A fundamental element when evaluating the optic nerve is detecting the scleral ring, which alerts the clinician to the optic disc size. Identifying a small optic nerve could lead to a diagnosis of optic nerve hypoplasia. This is especially important in pediatric patients since this condition can be associated with decreased vision, nystagmus, neurological defects, developmental delays, or endocrine abnormalities. No identifiable health information was included in this case report.
CASE REPORT
A Black woman aged 52 years presented for a 24-2 static threshold SITA-faster visual field and color vision testing after being diagnosed with left eye optic atrophy 2 months ago by another provider within the same facility.
Ocular history included left eye primary open-angle glaucoma diagnosed 7 years prior, for which she was prescribed timolol maleate 0.5% ophthalmic gel-forming solution once daily in the morning in the left eye. She was subsequently lost to follow-up after being diagnosed and she reported no longer using this medication. Medical history included type 2 diabetes mellitus, benign essential hypertension, vitamin D deficiency, chronic headache disorder, and obesity. Her medications consisted of empagliflozin 10 mg, combination losartan 50 mg and hydrochlorothiazide 12.5 mg, ergocalciferol 1250 mcg, and sumatriptan 50 mg.
Best-corrected visual acuity measured 20/20 in the right and left eye through -2.75-0.75x020 and -1.25-0.75x100, respectively. Pupil testing showed a left eye–positive relative afferent pupillary defect (log filters were not available for measurement). The Hardy-Rand-Rittler test indicated no color vision bundle defect in either eye. Extraocular motilities and confrontation visual fields were unremarkable. Slit lamp biomicroscopy recorded no evidence of anterior segment pathology in either eye. Intraocular pressures measured 19 mm Hg in each eye using Goldmann applanation tonometry. The previous dilated fundus examination by the other provider noted a smaller optic nerve size in both eyes, temporal pallor of the left optic nerve, and no signs of diabetic retinopathy. Color fundus photographs taken at this visit echoed those findings but also displayed an incomplete double ring sign from 7 to 11 o’clock in the left eye along with a lack of nasal retinal nerve fiber sheen (Figure 1).
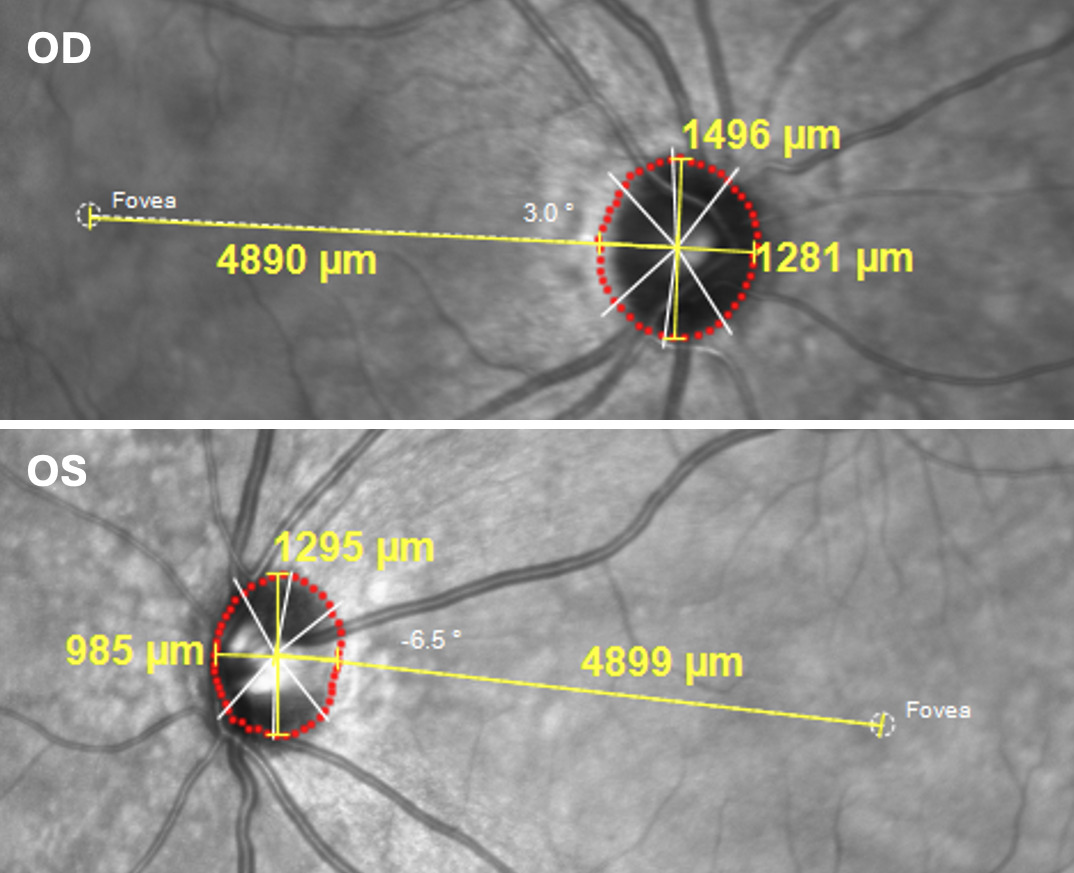
Spectral-domain optical coherence tomography taken at the previous examination and reinterpreted at this examination showed a temporal retinal nerve fiber layer shift of the right eye in the temporal-superior and temporal-inferior humps consistent with a myopic appearance of a more temporal optic nerve exit of the superior and inferior vasculature (Figure 2). Also apparent was nasal thinning of less than 1% compared with the reference database. The left eye retinal nerve fiber layer exhibited a split-bundle appearance in which the temporal inferior area shifted temporally, along with extensive nasal loss. Ganglion cell analysis of the right eye showed no areas of thinning (Figure 2). The left eye ganglion cell analysis, however, indicated thinning nasal to the macula in a pattern resembling a papillofoveal bundle defect with spillover into the papillomacular bundle, more so superiorly.1 The Bruch’s membrane opening area measured 1.5 mm2 and 1.02 mm2 in the right and left eyes, respectively (Figure 2). The disc-diameter to disc-macula ratio measured 3.52 in the right eye and 4.30 in the left (Figure 3).
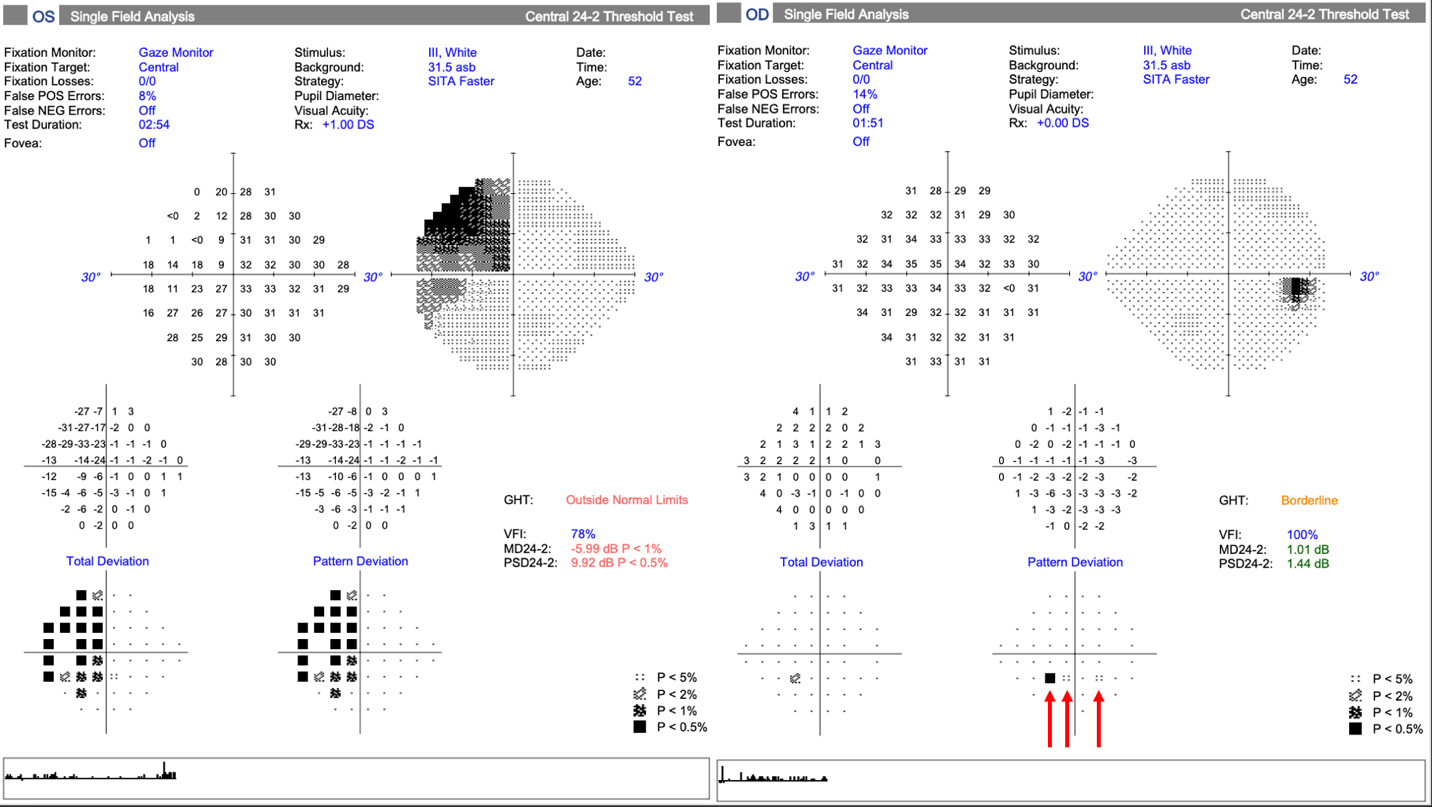
Visual field testing produced unremarkable results in the right eye, other than 3 points of pattern reversal likely attributable to the 14% false-positive rate (Figure 4). A dense superior temporal defect connected to the blind spot appeared in the left eye, which extended into the inferior temporal quadrant but respected the vertical midline (Figure 4). Though not available for review, the written assessment of the prior visual field examination also documented a left eye “temporal defect, more dense superiorly.”
At this point, nasal hypoplasia of the optic disc appeared to explain the left eye findings and the patient was initially diagnosed with this condition. Incidentally, at her primary care appointment 3 days prior to this examination, the provider ordered neurological imaging for the patient’s chronic headache disorder. This was completed several weeks after the patient was diagnosed with nasal hypoplasia of the optic disc. A review of the magnetic resonance imaging of the brain showed a reduced left optic nerve size compared with the right (Figure 5) and no optic nerve or chiasmal lesion. The magnetic resonance imaging also revealed an absent septum pellucidum, which led to the reclassification of the diagnosis from nasal hypoplasia of the optic disc to septo-optic dysplasia.
The patient exhibited no growth or developmental abnormalities. For completeness of care, endocrinology was notified through the internal medical facility messaging system to inform them of the recent diagnosis. They also noted an average adult female height of 162.56 cm and within reference range values of follicle-stimulating hormone, luteinizing hormone, thyroid-stimulating hormone, and basic metabolic panel. Per their direction, they suggested checking early morning cortisol levels, adrenocorticotropic hormone, insulin-like growth factor 1, free thyroxine, and a repeat thyroid-stimulating hormone. Those results were also within reference ranges. Neurology examined the patient for her chronic headache disorder after the magnetic resonance imaging. They mentioned the radiological and ophthalmic findings, concurred with the septo-optic dysplasia assessment, and suggested no further workup.
DISCUSSION
Septo-optic dysplasia is a rare congenital brain abnormality with varied reports of occurrence from 1 out of 10,000 live births to around 2 to 6 per 100,000.2–5 The classical septo-optic dysplasia triad consists of optic nerve hypoplasia, midline brain defects such as an absent septum pellucidum, and hypoplasia of the hypothalamo-pituitary axis.6 Septo-optic dysplasia is also known as de Morsier syndrome, which is an erroneous credit to the French-Swiss doctor believed to be the first to make the connection between the above findings.7 Though de Morsier coined the septo-optic dysplasia term, David Reeves first recognized this association at the Children’s Hospital in Los Angeles in 1941.7
To diagnose septo-optic dysplasia, it is generally accepted that only 2 of the 3 findings need to be present.2,4 In this case, both optic nerve hypoplasia and an absent septum pellucidum appeared, whereas the hypothalamic-pituitary axis remained unaffected. Only 30%-47% will fulfill all 3 criteria but nearly 95% have optic nerve hypoplasia.4,6 The average disc-diameter to disc-macula ratio in these patients is 3.25±0.26 as reported by Wakakura and Alvarez in their seminal work on the subject.8 In patients without septo-optic dysplasia, they found an average disc-diameter to disc-macula ratio of 2.67±0.19. A later study found a ratio of 2.54±0.13.9 However, those authors simply doubled the horizontal disc diameter vs halving the sum of the horizontal and vertical disc diameters.
When involved, the most common endocrinologic abnormality is growth hormone insufficiency.10 Other deficiencies include adrenocorticotropic hormone or cortisol, thyroid-stimulating hormone, and gonadotropin, which stimulates the pituitary gland to release follicle stimulating hormone and luteinizing hormone.11 Diabetes insipidus (not mellitus) may also occur and delayed or precocious puberty is common.12 Developmental delays and intellectual disability appear in half to two-thirds of cases, and more than one-third experience seizures and autism spectrum disorders.13
Radiological findings in septo-optic dysplasia vary and may include abnormalities other than the more frequently seen absent septum pellucidum. These include corpus callosum agenesis or hypogenesis (linked to development delays in children), pituitary infundibulum and/or gland hypoplasia, optic chiasm hypoplasia, and an altered appearance of the frontal horns of the lateral ventricles described as “point down or box-like”.2,7 Because nonmidline brain defects can occur, some advocate to abandon the septo-optic dysplasia term as its use emphasizes the septum pellucidum and diverts attention away from the overall complexity of the disorder.7 Others suggest the term “septo-optic dysplasia plus” to represent those with nonmidline brain defects, such as hydrocephalus, polymicrogyria, schizencephaly, white matter hypoplasia, and cortical heterotopia.6,7
An exact cause for septo-optic dysplasia remains elusive. Embryological development of the optic nerve and its extension to the chiasm begins and ends at about 6 and 8 weeks, respectively. This roughly coincides with corpus callosum and septum pellucidum development, which may explain the coexisting anomalies.5 Researchers identified the first transcription factor (HESX1) associated with septo-optic dysplasia in 1998, with several others joining the list years later (SOX2, SOX3, OTX2).3 Other potential contributors include maternal diabetes, young maternal age, primiparity, environmental factors such as drug or alcohol use, and viral infections, along with a few reports of familial cases.6 Interestingly, reports exist of a potential association between septo-optic dysplasia and a familial exudative vitreoretinopathy phenotype.14 Familial exudative vitreoretinopathy is an inherited retinal disorder with features including an avascular temporal peripheral retina, neovascularization, and retinal detachment.15 Therefore, clinicians diagnosing familial exudative vitreoretinopathy should perform a peripheral retinal examination.
Whether familial exudative vitreoretinopathy exists or not, recognizing optic nerve hypoplasia alone is critical as the American Association of Pediatric Ophthalmology and Strabismus recommends a magnetic resonance imaging and endocrine evaluation at the time of diagnosis.12 In a normal adult, this workup can likely be deferred. In this case, the radiological imaging was already scheduled by the primary care provider for the patients’ chronic headache disorder. Clinical findings in familial exudative vitreoretinopathy include normal to no light perception vision, nystagmus (bilateral hypoplasia), strabismus (unilateral hypoplasia), relative afferent pupillary defect, tortuous retinal vessels, and, of course, optic nerve hypoplasia with or without the double ring sign.4,13 Also, the hypoplasia does not need to be all-encompassing as several segmental conditions exist.
In superior segmental optic hypoplasia (a condition first reported by Petersen and Walton in 1977) only the superior optic disc is affected.16,17 Kim and colleagues coined the superior segmental optic hypoplasia term and described 4 features to the condition: Superior central retinal artery entrance, superior optic disc pallor, superior peripapillary halo, and superior thinning of the retinal nerve fiber layer.17 Of concern is the resemblance of this condition to glaucomatous optic neuropathy due to the superior retinal nerve fiber layer thinning and corresponding inferior temporal visual field defects. However, the authors cite the stability of the visual field defect and its connection to the blind spot as distinguishing superior segmental optic hypoplasia from glaucomatous findings.16
Another segmental hypoplasia—nasal hypoplasia of the optic disc—was initially diagnosed in the left eye in this case. First described in 1981 as a unilateral or bilateral condition, the constellation of findings of nasal hypoplasia of the optic disc include a nonprogressive temporal wedge visual field defect, a nasal entrance of the central retinal artery, nasal retinal nerve fiber layer thinning, and reduced nasal retinal vasculature.18 Although nasal retinal nerve fiber layer thinning of the other eye might infer bilateral nasal hypoplasia of the optic disc, the right eye failed to display the other characteristic findings.
CONCLUSION
Identifying optic nerve hypoplasia early on leads to timely intervention. Vision can be normal or severely reduced, and nystagmus or strabismus may be present. Newly diagnosed children should receive a magnetic resonance imaging and undergo endocrinological testing according to the American Association of Pediatric Ophthalmology and Strabismus.
TAKE HOME POINTS
-
It is paramount when examining the optic nerve to notate its size to assess for a hypoplastic disc, especially in children.
-
A magnetic resonance imaging and endocrinological testing should be ordered on every child diagnosed with optic nerve hypoplasia, with an increasing concern for septo-optic dysplasia with a bilateral and severe process.
-
If the radiological findings suggest septo-optic dysplasia, these patients may also have developmental delays, intellectual disability, seizures, autism spectrum disorders, and endocrinological abnormalities, of which the most common is growth hormone insufficiency.


_contrast-_and_brightness-enhanced_color_fundus_photograph_of_the_left_eye_highlights_t.png)
_right_and_(b)_left_eyes.png)


_axial_t2_flair_shows_an_absent_septum_pellucidum._if_present__it_would_show_as_a_thin_.png)


_contrast-_and_brightness-enhanced_color_fundus_photograph_of_the_left_eye_highlights_t.png)
_right_and_(b)_left_eyes.png)
_axial_t2_flair_shows_an_absent_septum_pellucidum._if_present__it_would_show_as_a_thin_.png)
