INTRODUCTION
Hypertension affects millions of people worldwide and hypertensive retinopathy is a well-known complication. Retinal findings include arteriolar narrowing, retinal hemorrhages, hard exudates, and cotton wool spots. Retinal neovascularization from severe retinal ischemia from malignant hypertension is extremely rare. A case of proliferative hypertensive retinopathy due to malignant hypertension secondary to primary hyperaldosteronism will be discussed. No identifiable health information was included in this case report.
CASE PRESENTATION
A 40-year-old White male was evaluated for a comprehensive eye examination during inpatient rehabilitation for a hemorrhagic stroke. The patient had a history of malignant hypertension secondary to primary hyperaldosteronism complicated by a hemorrhagic stroke and left hemiplegia.
The patient initially presented to the emergency department with an untreated blood pressure of 221/140 mm Hg. He was prescribed multiple antihypertensive medications with poor compliance and response. Because of uncontrolled chronic hypertension, he presented to the emergency department again 2 years later with a blood pressure of 211/114 mm Hg and experienced a hemorrhagic stroke. During the stroke workup, he was diagnosed with primary hyperaldosteronism from an aldosterone-producing adenoma. He underwent left adrenalectomy after the stroke to control his blood pressure. Postsurgery, his blood pressure was 177/125 mm Hg and gradually reduced to 130/90 mm Hg with multiple antihypertensive medications. He was also diagnosed with acute kidney injury at his initial emergency department visit that later developed into chronic kidney disease from malignant hypertension. The patient reported a social history of moderate tobacco and alcohol use, but the exact quantity of use is not known.
The patient had no significant ocular history before the stroke. After the stroke, the patient had been noted to have an incomplete left homonymous hemianopia and bilateral optic atrophy.
At his follow-up eye visit, 24 months after the stroke, the patient denied any vision changes. His blood pressure that morning was measured at 138/86 mm Hg. Best-corrected visual acuities were 20/30 in the right eye and 20/25 in the left eye. Extraocular motilities were normal, and there was no relative afferent pupillary defect in either eye. Confrontation visual fields were consistent with previous visual field results. All anterior segment findings were unremarkable for both eyes, including no iris neovascularization. Intraocular pressures were 16 mm Hg in the right and left eyes as measured by Goldmann applanation tonometry. Fundus examination of the right eye revealed diffuse pallor of the optic nerve with a 0.70 cup-to-disc ratio. Fundus examination of the left eye showed diffuse pallor of the optic nerve with a 0.55 cup-to-disc ratio. Both eyes had arteriovenous nicking with significant arterial attenuation greatest inferior. Both eyes also had scattered intraretinal hemorrhages with no exudates. Retinal neovascularization along the inferior temporal arcade and nasal quadrants without vitreous hemorrhage or significant fibrous traction was visible greater in the right eye than left eye.
Fundus photography of the right and left eyes showed optic nerve pallor and arteriovenous nicking with significant arterial attenuation greatest inferior, scattered intraretinal hemorrhages, and areas of retinal neovascularization greatest inferior and nasal in the right eye greater than the left eye (Figure 1).
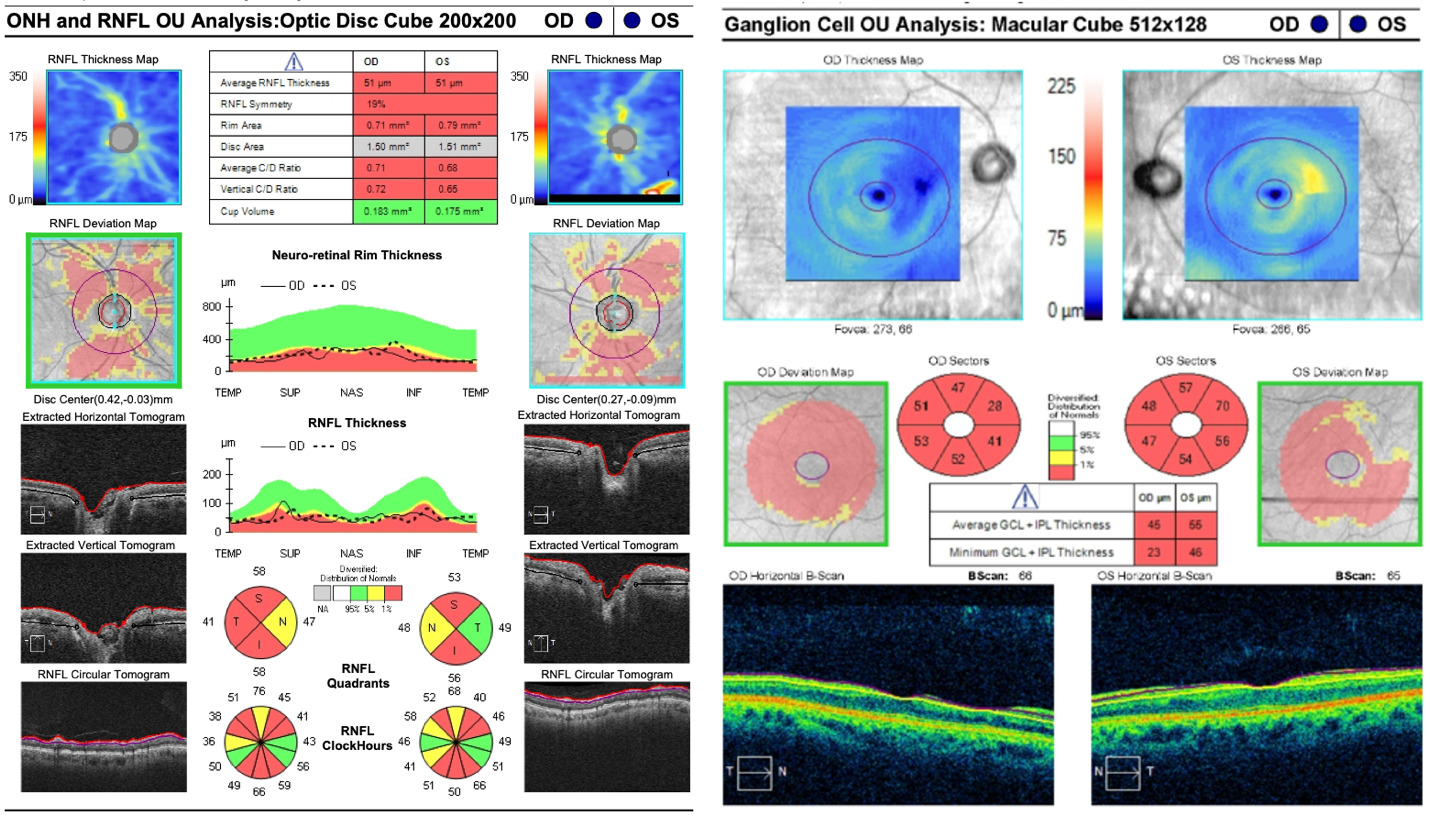
Optical coherence tomography of the retinal nerve fiber layer and ganglion cell-inner plexiform layer were completed (Figure 2). The retinal nerve fiber layer scan showed diffuse thinning in both eyes consistent with the optic nerve pallor seen on examination. The optical coherence tomography of the macula with ganglion cell analysis showed diffuse thinning of the ganglion cell-inner plexiform layer in both eyes. Spectral domain optical coherence tomography scans of the retina in both eyes confirmed the retinal neovascularization that extended into the vitreous cavity and was densest in the inferior and nasal quadrants. Optical coherence tomography angiography of both eyes revealed areas of ischemia, indicating capillary nonperfusion with adjacent retinal neovascularization that was greatest inferior (Figure 3).
Wide-field fluorescein angiography was performed on the right and left eyes (Figure 4). The right eye showed inferior, temporal, and nasal nonperfusion with multiple areas of retinal neovascularization. The left eye revealed multiple areas of nonperfusion and nasal and inferior retinal neovascularization. There was no delay in vein filling noted. The patient received panretinal photocoagulation in both eyes and achieved regression of retinal neovascularization.
DISCUSSION
There are very few case reports of retinal neovascularization associated with systemic hypertension reported in the literature. Proliferative retinopathy can be seen in systemic diseases that lead to retinal ischemia, including diabetes mellitus, sickle cell disease, sarcoidosis, and lupus erythematosus.1 The patient in this case report had a history of malignant hypertension secondary to primary hyperaldosteronism, and detailed history and extensive systemic and ophthalmic workup failed to reveal an alternative explanation for his proliferative retinopathy. The patient was followed by endocrinology and received multiple complete blood counts with differentials revealing normal levels of hemoglobin, hematocrit, platelet count, and white blood cell count. Patient had normal levels of vitamin B12, folate, and hemoglobin A1c. Other laboratory testing included HIV and antinuclear antibody, which were negative. Chest x-ray did not show signs of sarcoidosis or tuberculosis. The ophthalmic ancillary testing revealed diffuse retinal ischemia and retinal neovascularization. Based on the workup, there was no evidence of vasculitis from infectious or inflammatory etiologies, no hemoglobinopathies, no diabetes, and no history of drug abuse as a cause for his retinal neovascularization.
Neovascularization and fibrovascular proliferation of retinal vessels is a response to retinal ischemia and an upregulation of growth factors.2 There are only 5 published case reports of retinal neovascularization associated with uncontrolled hypertension, and 4 of these include young patients in their 30s to 40s, like the patient in this case report (Table 1).3–7 All the cases had retinal findings consistent with hypertensive retinopathy, including retinal ischemia and neovascularization.
In a case presented by Georgiadis et al in 2016, the patient was a 33-year-old male with reduced vision and retinal neovascularization, presumed to be from hypertension secondary to hyperaldosteronism.3 This case is similar to the patient in this case report as they are both young male patients with malignant hypertension secondary to hyperaldosteronism. Aldosterone is a mineralocorticoid that acts on sodium channels in the collecting tubules of the kidney and causes sodium reabsorption. When there is too much aldosterone, the increased reabsorption of sodium leads to hypertension. The patient in this case study had an aldosterone-producing adenoma, which is the most prevalent cause of primary hyperaldosteronism.8 Although, primary hyperaldosteronism can cause elevated blood pressure, primary aldosteronism is typically associated with low plasma renin levels, whereas malignant hypertension is typically the opposite and associated with high plasma renin levels. These two conditions are on opposing ends of the renin spectrum; therefore, it is rare to have findings of malignant hypertension from primary aldosteronism, especially from a unilateral aldosterone-producing adenoma.9 The etiology of the malignant hypertension in this case is unique because the blood pressure was extremely high and chronically elevated. This might explain why most patients with primary hypertension do not develop proliferative hypertensive retinopathy, as the blood pressure never reaches a level that leads to widespread ischemia. Rapid persistent increase in blood pressure damages the precapillary retinal arterioles, leading to smooth muscle necrosis and failure of the autoregulation. This will cause endothelial damage and breakdown of the blood retinal barrier, along with microvascular occlusion, which results in leakage and ischemia.10,11 This severe retinal ischemia could then cause an increase in angiogenic factors resulting in retinal neovascularization.
Other case reports in the literature on proliferative hypertensive retinopathy included patients with immunoglobulin A nephropathy and uncontrolled chronic primary hypertension.4–7
For the patient in this case report, the proliferative retinopathy was identified early before the development of significant vision complications. Other case reports had patients with severe retinopathy and significant vision loss.3–7 Proliferative retinopathy can become visually devastating from severe macular ischemia, tractional retinal detachments, or neovascular glaucoma.3–7 Treatment includes pan retinal photocoagulation and anti–vascular endothelial growth factor therapies.3,5–7 Most patients in the published case reports demonstrated regression of the retinal neovascularization after treatment, similar to the current case.
CONCLUSION
Proliferative hypertensive retinopathy is rare, with only a few reported cases.3–7 This case is unique because the retinal neovascularization was due to hypertensive ischemic retinopathy from malignant hypertension secondary to primary hyperaldosteronism from a unilateral aldosterone-producing adenoma. It is important to recognize this uncommon complication as a cause of proliferative retinopathy.
TAKE HOME POINTS
-
Hypertension is an uncommon cause of retinal neovascularization.
-
In patients with retinal neovascularization, it is important to get a detailed history and systemic workup to rule out other causes of neovascularization.
-
Early diagnosis and treatment of hypertension and ocular complications is important to limit significant visual complications.