INTRODUCTION
Glaucoma features progressive, irreversible ganglion cell and nerve fiber loss and, ultimately, field loss if intraocular pressure is inadequately modulated.1 Intraocular pressure fluctuation can refer to variation in pressure measurements over the course of 24 hours or serial office visits. Pressure fluctuates more in glaucomatous than healthy eyes.2 Even with treatment, pressure fluctuation has been associated with glaucomatous field progression, particularly in patients with low mean pressure.3
In assessing glaucoma progression speed, trend-based analysis provides actual rate of change.4 Visual field index, an aggregate of remaining field, is one method to categorize progression.5 More than 1% per year may be fast progression, with approximately 0.5% per year average for a treated patient with glaucoma.5,6 Na et al showed that glaucomatous eyes with progressive field loss had mean circumpapillary retinal nerve fiber layer loss of -1.26 µm/year.7 Average ganglion cell–inner plexiform layer progression in 451 treated eyes with primary open-angle glaucoma was -0.231 μm/year.8 Macular ganglion cell complex loss faster than -1.3 µm/year has been associated with uncontrolled glaucoma progression.9
Angle-closure glaucoma, characterized by contact between the iris and trabecular meshwork, can take place acutely, subacutely, or chronically.10 It can be associated with severe to mild or absent symptoms.11 Asymptomatic intraocular pressure fluctuations in partial or subacute angle closure can be missed or misdiagnosed, resulting in progressive glaucomatous damage.10,11 Furthermore, not all eyes will display peripheral anterior synechiae after closure.12 It is important not to miss an angle-closure component in glaucoma cases because it responds significantly better to laser/surgical treatment. Meanwhile, selective laser trabeculoplasty is not indicated in untreated angle-closure cases.13
Gonioscopy is the gold standard when assessing angle-closure risk. Shaffer developed a commonly used grading system whereby the angle at which the iris inserts relative to the trabecular meshwork is approximated.14 Anterior segment ultrasound biomicroscopy angle width grading correlates linearly with this gonioscopy grading.15,16 According to the Shaffer grading system, angles of 20 degrees or less are capable of closure. The risk of significant angle closure becomes exponentially higher when the angle is 20 degrees or less. Angles of 10 degrees or less are associated with probable closure risk.17 The modified Shaffer classification system corresponds angle width in degrees to the most posterior visible angle structure without indentation.18 Ten degrees corresponds to Schwalbe’s line only (grade 1); 20 degrees to trabecular meshwork (grade 2); 20 to 35 degrees to scleral spur (grade 3); and 40 degrees to ciliary body (grade 4).19 Assessing 360 degrees of the angle altogether, an occludable angle may be defined as lack of visualization of posterior trabecular meshwork for greater than or equal to 180 degrees without indentation.20
Gonioscopy is dependent on examiner experience and proficiency and involves the use of a topical anesthetic.21 Anterior segment ultrasound biomicroscopy also involves significant skill, topical anesthetic, possible patient discomfort, and supine patient positioning.15,16 Clinicians may therefore be inclined to use noncontact angle assessment tests such as the Van Herick technique, anterior segment optical coherence tomography, and Scheimpflug imaging.21 The longer wavelength of swept source optical coherence tomography reduces scattering and enhances penetration relative to spectral domain.22 By following the boundary between the longitudinal fibers of the ciliary muscle (dark) and the sclera (white) until it reaches the anterior chamber, the scleral spur is identifiable. Angle closure is observable as touch between the iris and angle wall anterior to the scleral spur.23 The Van Herick limbal chamber depth technique can be graded as a fraction of the adjacent most peripheral temporal cornea/limbus, then expressed as a ratio. Although this technique is widely used, there is evidence of this technique missing a not insignificant number of occludable angles relative to gonioscopy assessment.24–26
Practitioners may also have access to Scheimpflug imaging, for example, the Pentacam HR (Oculus, Wetzlar, Germany). Pentacam imaging combines a rotating Scheimpflug camera with a static camera to acquire up to 50 cross-sections through the cornea and anterior chamber/angle. It is quicker and requires significantly less skill than ultrasound or gonioscopy to perform.27 From the slices, a 3-dimensional model of the anterior segment is formed. From this model, “the smallest chamber angle calculated from the 3D model” is the reported angle width. Anterior chamber depth and volume are also provided.28,29
CASE REPORTS
Case 1
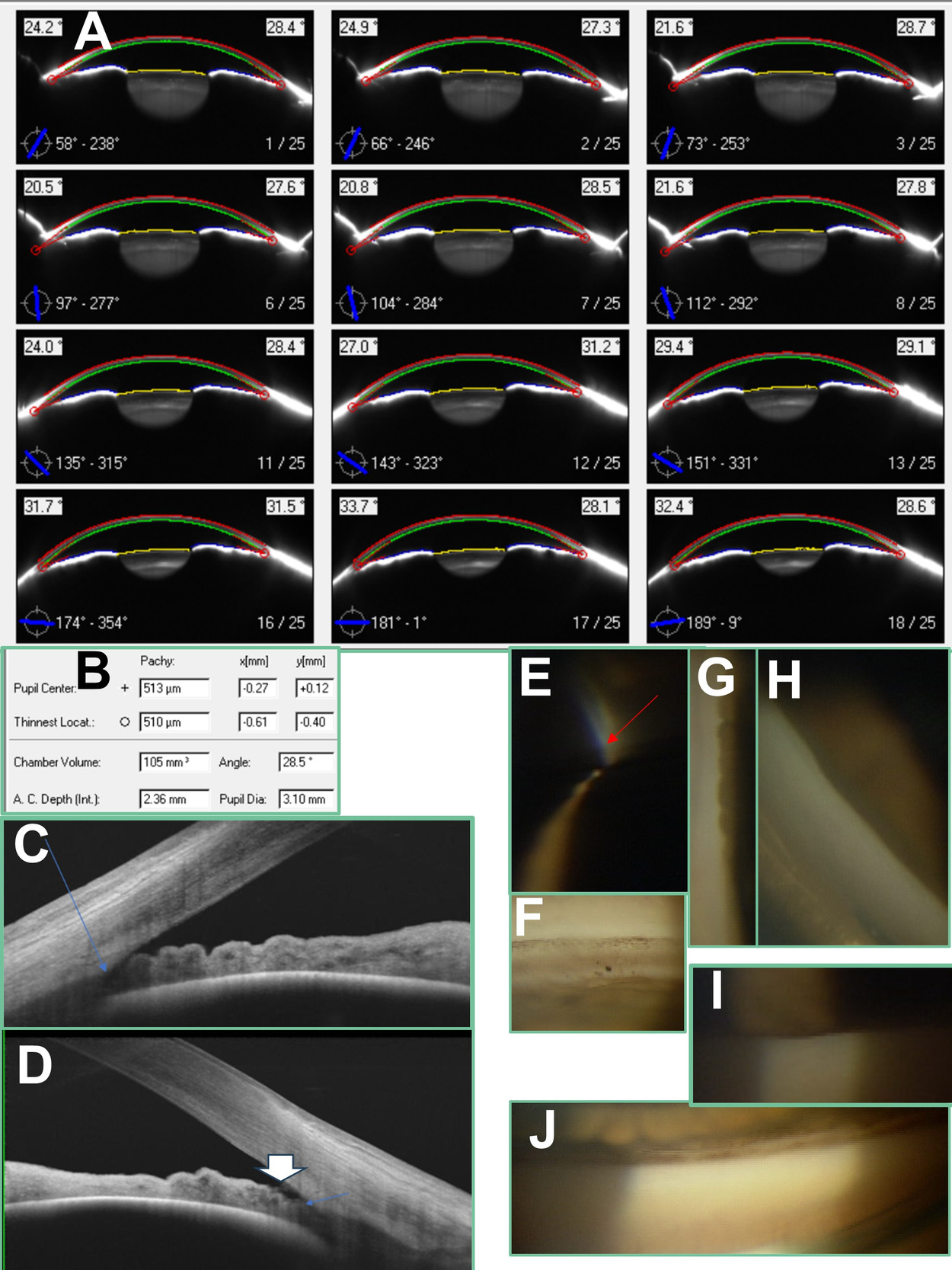
A 65-year-old White man was referred for selective trabeculoplasty consideration at a university teaching clinic. He had a history of right eye trauma and subsequent no light perception vision and mild hyperopia with excellent corrected acuity in the left (Table 1). For cosmesis he wears a custom hand-painted Kontur soft contact lens and has regular visits in the contact lens clinic. Record review revealed he was diagnosed with primary open-angle glaucoma in his left eye 3 years prior with superior rim thinning/cupping and corresponding nerve fiber defect (Figure 1A). This also correlated with a dense inferior nasal step/partial arcuate field defect, as shown in the guided progression analysis baselines in Figure 1E. Pentacam report showed angle width of 32 degrees in the dark and lower-than-average chamber volume and depth and central corneal thickness in his left eye (Table 1; Figure 2A-C). Maximum left intraocular pressure prior to treatment measured 23 mmHg. Dorzolamide twice daily was added to latanoprost 4 months after treatment initiation. For 2.5 years, his pressure has ranged from 10 to 14 mmHg in his left eye in office, checked roughly every 4 months. Systemic workup was negative for nocturnal blood pressure dip, sleep apnea, and anemia. Despite the compliance and reasonable in-office intraocular pressure measurements, a possibly significant loss of rim area on disc topography relative to baseline (0.40 versus 0.48 mm2) was observed approximately since treatment initiation (Figure 1B). Fast nerve fiber layer (-1.14 µm/year) and ganglion cell–inner plexiform (-1.24 µm/year) progression was also measured (Figure 1C, D). Visual field index trend was also fast at greater than -1% per year but did show a wide confidence interval, as shown at the bottom of Figure 1E. As a result of the still “open” angle width on Pentacam and concern over the speed of progression in the left eye despite treatment compliance, he was referred from contact lens service for selective laser trabeculoplasty consultation.
At consultation, intraocular pressure measured 12 mmHg in the left eye. Van Herick angle assessment measured 1:0.25 (grade 2). Darkroom gonioscopy showed the left eye open to Schwalbe’s line without indentation inferiorly and superiorly (Figure 2F, J). Nasal and temporal angles appeared open to anterior trabecular meshwork with mild to moderate lens tilt (Figure 2H); indentation revealed the more posterior trabecular meshwork portion as well as iris processes (Figure 2I). Indentation also showed a double hump iris pattern, increasing suspicion of plateau iris (Figure 2G). Anterior segment optical coherence tomography corroborated the angle narrowness, with iridocorneal touch suspected anterior to the scleral spur (Figure 2D, E). It also revealed a planar, rather than convex, iris configuration and unimpressive lens vault, possibly consistent with plateau iris. He denied symptoms (headaches, eye pain, haloes around lights, nausea) of acute angle closure when asked. He agreed to a referral to a tertiary surgical center for anterior segment ultrasound biomicroscopy and second opinion on best intervention: peripheral iridotomy and/or cataract surgery and/or iridoplasty given the suspicion for a plateau iris contribution to his glaucoma. Ultrasound biomicroscopy revealed plateau iris, and the surgeon agreed that intermittent angle closure was possible based on gonioscopy. The patient and surgeon ultimately opted for early cataract surgery to open the anterior chamber depth. Intraocular pressure measured 11 mmHg in the left eye on latanoprost alone several months after the procedure.
Case 2
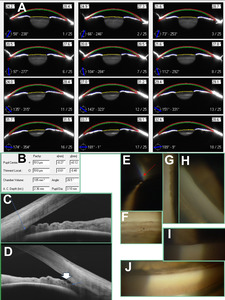
A 65-year-old White woman with history of bilateral pseudoexfoliation was internally referred for selective laser trabeculoplasty consideration. She had a history of symptomatic ocular surface disease secondary to ocular rosacea managed in the university dry eye clinic. Record review showed significant superior and inferior rim thinning both clinically and on optical coherence tomography disc topography at baseline 2 years prior (Figure 3A). This correlated with nerve fiber layer loss in the same locations as well as diffuse macular ganglion cell–inner plexiform loss. A superior nasal step was prominently measured by the second baseline field (Figure 3E). Intraocular pressure prior to treatment initiation measured in the mid-20s in the right and high-teens in the left. Lower-than-average corneal thickness was measured on Pentacam (Figure 4B). She was started on Zioptan (tafluprost 0.0015%) bilaterally and preservative-free dorzolamide 2%–timolol 0.5% in the right eye after the above imaging baselines. Despite intraocular pressure over the next 2 years measuring in the low-teens in both eyes, a significant amount of rim loss and rate of nerve fiber progression (-2.36 µm/year) was measured in the right eye (Figure 3B, C). The left eye was stable. After moving the right eye’s ganglion cell–inner plexiform layer baseline to a higher-quality scan from a later examination, a fast rate of progression was observed at -1.48 µm/year (Figure 3D). Visual field index trend analysis showed a potentially very worrying slope of -4.4% per year, although the wide confidence interval of ±7.8% per year was acknowledged (Figure 3E). Record review of her dry eye treatment showed no topical steroid usage. Pentacam angle assessment at the latest dry eye clinic visit showed mean angle measurements of 28 and 33 degrees in the right and left eyes, respectively (Figure 4A, B). This still “open” angle on Pentacam and well-founded concern over glaucoma progression speed prompted a referral for trabeculoplasty of the right eye.
At consultation, intraocular pressure measured 13 mmHg in each eye. Van Herick angle assessment measured grade 1 1: 0.25 (grade 2) in the right and 1: 0.5 (grade 3) in the left. There was no iris flutter or iridodonesis, nor was there clinically visible lens subluxation observed in either eye in different gaze positions. Darkroom gonioscopy avoiding excessive lens tilt or pressure showed angles open to just about posterior trabecular meshwork inferiorly and anterior meshwork/Schwalbe’s line in the other 3 quadrants (Figure 4E, H, I). Moderate lens tilt was required to obtain a view of the angle over the hill of the convex iris (Figure 4G). Indentation confirmed 3+ patchy posterior trabecular meshwork pigmentation in the right eye inferiorly, consistent with pseudoexfoliation syndrome, and just about revealed the entirety of the posterior meshwork and scleral spur 360 degrees. Relative to the right eye, gonioscopy on the left eye showed angles significantly more open to scleral spur and ciliary body band (Figure 4F, J). Swept-source anterior segment optical coherence tomography showed near iridotrabecular touch anterior to the scleral spur in the right eye, particularly in the noninferior quadrants (Figure 4C). The inferior angle was slightly more open, with exfoliative debris between the iris and trabecular meshwork observed (Figure 4D). Instead of laser trabeculoplasty, the patient agreed to tertiary referral for an opinion on cataract extraction with anterior chamber washout to reduce exfoliative debris along with concomitant microinvasive glaucoma surgery. The surgeon agreed that an angle-closure component was likely contributing to progression, and the patient consented to cataract surgery. Gonioscopy-assisted transluminal trabeculotomy was performed with the surgery to further lower medication burden. Several months after the procedure, intraocular pressure measured 10 mmHg in the right eye and 13 mmHg in the left eye on tafluprost 0.0015% bilaterally.
DISCUSSION
Given the Pentacam anterior chamber angle width report features the smallest angle width obtained from the Scheimpflug cross-sections,28,29 this width would ideally correlate with our smallest angle quadrant seen on gonioscopy. For the left eye in case 1, the narrowest gonioscopy angle quadrant showed Schwalbe’s line (equating to 10 degrees based on Shaffer grading), but the angle width on the Pentacam report measured 32.1 degrees. For the right eye in case 2, the narrowest gonioscopy angle measurement recorded was Schwalbe’s line/anterior meshwork equating to 10 to 20 degrees. This was also not insignificantly less than the 28.5-degree angle width shown on the Pentacam report. From an overall angle perspective, both of these eyes had “occludable” angles or at least 180 degrees of gonioscopy grading where the filtering meshwork was not seen. The optical coherence tomography imaging in both cases showing possible iridotrabecular contact or near touch anterior to the scleral spur also supports closure risk (Figure 2D, E; Figure 4C). These cases highlight the potential for Pentacam angle width report measurements to offer false reassurance against angle closure, likely contributing to glaucomatous progression.
In a study of 72 eyes of various angle widths, Kurita et al found that Pentacam anterior chamber volume (r = 0.81) and depth (r = 0.85) correlated more with grade of narrowing on Shaffer gonioscopy grading than Pentacam angle width (r = 0.65).15 In the 43 eyes with a Shaffer’s grade of 2 or less, the corresponding ultrasound biomicroscopy angle width measurement was not correlated with Pentacam angle width (r = -0.13).15 Angle width measurements via optical coherence tomography and ultrasound biomicroscopy show good correlation with gonioscopy grade.15,16,30 However, width correlation with gonioscopy may not be as strong for Scheimpflug imaging, particularly in narrow angles. This is likely due to total internal reflection causing unreliable localization of the angle apex with Scheimpflug imaging.15,16 Therefore, only the angle approach can be imaged, with the location of the angle recess only estimated.16 Furthermore, the eyelid may obstruct acquisition of the Scheimpflug angle image, resulting in the need for further extrapolation to obtain angle width measurements in certain slices.28,29 Shajari et al observed that Pentacam angle width measurements corresponded to a higher score in the Shaffer grading system for a given angle when said angle was actually viewed on gonioscopy. Shajari et al subsequently cautioned that using Pentacam angle width measurements alone may result in an underestimation of the likelihood of angle closure.31 Pakravan et al proposed less than 27 degrees for Pentacam angle width as a threshold for high risk for angle closure, as this seemed to correlate with posterior meshwork not being visible in 3 gonioscopy quadrants.32 Note that the Pentacam report’s angle grading for case 2 matched up close but just higher than this threshold at 28.5 degrees. Meanwhile, case 1’s Pentacam grading of 32.1 degrees was still significantly higher. We wonder whether the relatively flat iris in case 1’s plateau iris configuration might have contributed to an even greater automated angle overestimation/extrapolation from the Pentacam. Kurita et al also expressed concern that plateau iris angle narrowing in particular could be overlooked by Pentacam.15
Kurita et al noted that a central anterior chamber depth of 2.58 mm had 100% sensitivity and 87% specificity in separating angle-closure eyes from closure-suspect eyes based on gonioscopy assessment.15 Note that case 1 had a chamber depth of 2.59 mm, right on this cutoff, whereas case 2 was below at 2.36 mm. Reproducibility of the Pentacam chamber depth and volume have been noted as higher than the angle width parameter.15,16 Interestingly, Kurita et al found that chamber volume was the most successful individual parameter in diagnosing angle closure despite this parameter still requiring some data extrapolation for the angle recess portion of its measurement.15 Grewal et al found that using a chamber volume cutoff of 113 mm3 provided 90% sensitivity and 88% specificity for detecting closed angles and actually outperformed several well-established parameters on anterior segment optical coherence tomography.33 Note that the right eye in case 2 was below this chamber volume cutoff (105 mm3), whereas the left eye in case 1 was above (136 mm3). Note that Grewal et al did not evaluate the usefulness of the Pentacam angle width parameter, stating its “reliability in eyes with narrow angles has been questioned,” as discussed above.33 Other authors have noted relatively better performance of Pentacam angle width; however, their results also showed that angle width performed worse than chamber volume and depth in differentiating open angles from those with closure.34,35
Symptoms in subacute angle closure are milder than those in acute angle closure or can be absent. This condition has also been called intermittent, prodromal, or subclinical angle closure. Relative to 360-degree closure seen in acute attacks, subacute attacks may have only partial closure.10,11 Some patients might have symptoms such as headache and eye pain that are so mild they are attributed to other causes; the patient can often go to bed and wake up with the symptoms resolved.11 Furthermore, there is a marked variation in the tendency of eyes to form synechiae, with anterior synechiae not forming in some eyes despite repeated closure.12 Some eyes with angle-closure glaucoma “run a completely asymptomatic course” to blindness unless appropriate diagnosis and intervention is made.10,11 Varma et al state that the angle closure they encounter in a tertiary glaucoma clinic is often asymptomatic and chronic. Further, a significant number of narrow-angle glaucoma cases are misdiagnosed as open-angle glaucoma, mandating the need for proper, serial gonioscopy examinations in patients.1
Notably, the amount of diurnal pressure fluctuation as well as glaucomatous progression also appears to increase with greater degree of angle narrowing in moderate-sized studies of approximately 100 patients.36–38 Given the progression of the left eye in case 1 and right eye in case 2 despite multiple topical medication classes and seemingly good pressure control in office, these eyes might have been suffering from significant, damaging intraocular pressure fluctuations outside of office. However, acknowledging the lack of symptoms and anterior synechiae in both cases, these 2 eyes’ continued progression cannot definitively be attributed to pressure fluctuations from intermittent angle closure. Nevertheless, the magnitude of angle closure–induced pressure elevation may have been blunted by regular aqueous suppressant drop usage. Intraocular pressure may have presumably been above the low-to-mid-teen target pressure but not high enough to induce significant corneal edema to result in haloes/visual blur or symptoms of ischemia to the intraocular structures to elicit ocular pain.39
Nocturnal drops in ocular perfusion pressure due to blood pressure dipping and/or nocturnal eye pressure spikes unrelated to angle closure might also have contributed toward progression.40 We did not measure the patients’ 24-hour blood or eye pressures. Furthermore, although topical glaucoma medications such as latanoprost are thought to lower 24-hour intraocular pressure variability, it has still been found that average pressure reduction control at night via latanoprost is worse than that obtained during the day. Meanwhile, the same study found that dorzolamide and timolol were not shown to lower intraocular pressure significantly at night.41 Although these 2 patients were suspected to be highly adherent, an upsurge of topical treatment compliance leading up to the office visits is yet another possibility given the ability of physicians to identify which patients are noncompliant toward topical medications is imperfect.42 Finally, it could be argued that the right eye in case 2 could have progressed due to the aggressive nature of pseudoexfoliative glaucoma alone. However, angle narrowing appears to be found in a significant number of exfoliative glaucoma eyes and may play a role (along with the trabecular accumulation of exfoliative debris) in the pathogenesis of pseudoexfoliative glaucoma and its poorer response to medical therapy versus primary open-angle glaucoma.43–46
CONCLUSION
We report 2 examples that may serve as a reminder that Pentacam Scheimpflug measurements may overestimate the angle width in high-risk patients on the narrower end of the angle width spectrum. In these 2 cases, anterior segment optical coherence tomography and particularly gonioscopy showed these eyes’ angles to potentially be significantly narrower than the Pentacam angle width report suggested. When using Pentacam to supplement other methods of angle assessment in patients with glaucoma or those suspected of having glaucoma, anterior chamber volume and depth may be more useful than angle width measurements. It is proposed that Pentacam angle width should not be used to rule out the possibility of intraocular pressure fluctuations associated with intermittent angle closure in patients with progressing glaucoma and narrow angles.
TAKE HOME POINTS
-
Pentacam Scheimpflug angle width measurement may misrepresent actual angle status in patients with narrow angles; instead, anterior chamber depth and volume may correspond better to more established measures of angle assessment.
-
Gonioscopy remains the gold standard for angle assessment in patients with glaucoma.
-
Intermittent angle closure may be worthwhile to consider as a differential cause of intraocular pressure fluctuations in phakic patients with progressing glaucoma despite low in-office intraocular pressure measurements.