INTRODUCTION
Anemic retinopathy is caused by retinal ischemia due to low red blood cell mass. It can present with retinal hemorrhages, cotton wool spots, and a generalized increase in overall retina thickness, including macular edema. In this case report, we present ocular findings and treatment associated with anemic retinopathy in a patient who experienced acute blood loss from a gastrointestinal bleed. Written informed consent was obtained for this case report. No identifiable health information was included in this case report.
CASE REPORT
A 57-year-old African American woman presented to the clinic with complaints of reduced near vision, photophobia, and headaches for approximately 1 week. She shared she was unable to read without a magnifier until 1 day prior, but her vision seemed to be improving. She denied flashes, new floaters, eye pain, and diplopia. She reported she was recently discharged from the hospital following an acute gastrointestinal bleed, and her visual symptoms started at that time. She also reported severe headaches during her hospitalization, and these were improving as well. On review of her hospitalization records, she had been admitted to the hospital for 3 days and was discharged 9 days prior to her presentation to the eye clinic. She was last examined 1 year ago and managed for mild nonproliferative diabetic retinopathy without macular edema and glaucoma suspicion based on optic nerve appearance.
The patient’s medical history included type 2 diabetes mellitus for 17 years. The patient’s most recent hemoglobin A1c (HbA1c) was taken 6 months prior and was 8.3%. The patient was also managed for hypertension, hyperlipidemia, and hypothyroidism. Of significance, the patient reported multiple hospitalizations over the past 3 months due to gastrointestinal bleeding, with the most recent hospitalization lasting 3 days. During this episode, the patient’s hemoglobin had dropped from 7.9 g/dL to 5.4 g/dL. Her platelet count was 255,000 platelets per microliter of blood. The patient declined a blood transfusion and was treated with erythropoietin 40,000 IU, intravenous iron infusions, B12 injections, and folate supplements. She was prescribed ferrous sulfate 325 mg daily and folic acid 1 mg daily on release from the hospital.
Entrance Snellen visual acuity in habitual glasses was 20/20-2 in the right eye and 20/20-2 in the left eye with a prescription of -0.25 -1.00 x090 right eye and -0.25 -1.25 x085 left eye. Manifest refraction revealed a mild hyperopic shift to +0.25 -1.00 x093 right eye and +0.25 -1.50 x088 left eye, with no improvement in best corrected visual acuity. Near vision was tested with both eyes. The patient preferred a +2.50 add, and near visual acuity was 20/20 both eyes with this add in place. Pupils were equal, round, and reactive to light with no relative afferent pupillary defect. Extraocular muscles were smooth and full without diplopia or pain. Confrontation visual fields were full to finger counting in both eyes. The anterior segment was unremarkable. The eyelids and conjunctiva were clear. The cornea was clear without any fluorescein staining. Anterior chambers were deep and quiet without cells or flare. The iris was flat and intact without neovascularization in both eyes. Intraocular pressure when measured by Goldmann applanation tonometry was 14 mm Hg in the right eye and 12 mm Hg in the left eye.
Both eyes were dilated using one drop of 1% tropicamide and 2.5% phenylephrine. The posterior segment evaluation revealed trace nuclear sclerosis of the lens of both eyes. The vitreous was clear and quiet. Optic nerve heads were pink with distinct margins with a cup-to-disc ratio of 0.55 round right eye and 0.60 round left eye. The posterior poles revealed flame hemorrhages with scattered intraretinal hemorrhages, cotton wool spots, and Roth spots in both eyes (Figure 1). The left eye revealed retinal hard exudates in the superior temporal macula. The peripheral retina was flat and intact in both eyes. Her blood pressure was measured right arm seated and was 146/82 mm Hg.
Optical coherence tomography of the macula and optic nerves was performed revealing intraretinal and subretinal fluid in the macula of both eyes (Figure 2). Both eyes showed diffuse retinal thickening around the optic nerve head (Figure 3).
The differential diagnoses were anemic retinopathy with macular edema in both eyes, bilateral central retinal vein occlusion with macular edema, and nonproliferative diabetic retinopathy with macular edema. The patient was referred for evaluation with a retina specialist for management of the macular edema.
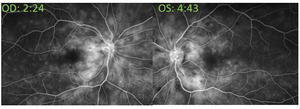
At the retina consultation, 4 days after initial presentation, visual acuity was 20/20 right eye and 20/20-1 left eye, and the patient subjectively felt her vision was improving. Intraocular pressures were 14 mm Hg right eye and 12 mm Hg left eye when measured by tonopen (with 95% confidence). Anterior and posterior segments were unchanged. Optical coherence tomography of the macula was performed and revealed resolution of the intraretinal fluid and resolving subretinal fluid in both eyes (Figure 2). Fluorescein angiography was performed and revealed normal transit time with mild leakage of both eyes without macular edema or hyperfluorescence of the optic disc. There were no distinct areas of nonperfusion in either eye. Both eyes showed engorged and mildly tortuous veins (Figure 4).
The retina specialist confirmed the diagnosis of anemic retinopathy with macular edema of both eyes based on the presence of retinopathy in the setting of acute anemia. Based on excellent vision and improving macular edema, continued monitoring was recommended. The patient was scheduled for a follow-up appointment in 4 weeks.
The patient presented 1 month later to the retina specialist. She reported normal vision and was no longer experiencing headaches and photophobia. Entering visual acuity was 20/20 in both eyes. Intraocular pressure measured with rebound tonometry was 15 mm Hg right eye and 14 mm Hg left eye. Anterior segment evaluation was unremarkable. Posterior segment evaluation revealed resolving flame hemorrhages near the optic nerves of both eyes, with remaining scattered intraretinal hemorrhages and cotton wool spots within the arcades. The macula of both eyes was flat with few scattered resolving hemorrhages. Optical coherence tomography of the macula showed resolved intraretinal and subretinal fluid in both eyes. Her hemoglobin was 11.6 g/dL. She was scheduled for a 4- to 6-month follow-up.
The patient was lost to follow-up; however, she presented to the optometry clinic 8 months after the initial presentation. The patient denied any current ocular complaints. Entering visual acuity through habitual glasses prescription of -0.25 -1.00 x090 right eye and -0.25 -1.25 x085 left eye was 20/20 in both eyes. Intraocular pressure measured with rebound tonometry was 15 mm Hg right eye and 14 mm Hg left eye. Anterior segments were unremarkable. Posterior segments revealed resolving scattered intraretinal hemorrhages with rare cotton wool spots in both eyes (Figure 5). The left eye had remaining exudates in the superior temporal macula. Optical coherence tomography of the macula showed resolved intraretinal and subretinal fluid in both eyes. The right eye had a normal foveal contour. The left eye showed inner retinal thinning nasal to the fovea (Figure 2) consequential to the resolution of cotton wool spots in the papillomacular region and resolution of the macular edema observed at the day 4 follow-up.
DISCUSSION
Anemia is a disorder characterized by a pathological reduction of the body’s red blood cell mass.1 Anemia is usually the result of 1 of 3 mechanisms: either by lack of production of red blood cells, red blood cell loss is greater than replacement, or the body destroys red blood cells faster than they can be produced.2,3 The 3 primary red blood cell measurements in assessing anemia include the red blood cell count, the hemoglobin concentration, and the hematocrit.1 Red blood cells are further described by their size using the mean corpuscular volume as either microcytic, normocytic, or macrocytic.3 Red blood cell color is assessed using a peripheral blood smear and can be described as either hypochromic (pale due to low hemoglobin concentration), normochromic (red), or hyperchromic (deeper shade of red due to high hemoglobin concentration). These physical attributes of the red blood cells yield clues to the type of anemia and, in conjunction with the reticulocyte count (immature red blood cells), can discern whether the body’s bone marrow compensatory response is adequate to the abnormal red blood cell mass.1,3,4
The World Health Organization defines anemia as hemoglobin less than 13 g/dL for men and less than 12 g/dL for women.4,5 A hemoglobin concentration between 10 and 12 g/dL is considered mild anemia, between 7 and 10 g/dL is moderate, and less than 7 g/dL is severe anemia.1 If the hemoglobin is less than 5 g/dL then the ability to deliver oxygen to the body’s tissues is dramatically reduced, and cognitive function and central neuronal processing may be impaired.6,7
In contrast to acute blood loss anemia as demonstrated in this case, the 2 most common types of anemia are iron deficiency anemia and anemia of inflammation, formerly known as anemia of chronic disease.3,4,8 Common symptoms of all anemias are nonspecific and may include fatigue, dizziness, headaches, palpitations, blurred vision, and tinnitus.1,4
Iron deficiency is the most common form of anemia worldwide.2 When the body’s supply of iron is low, not enough hemoglobin is produced. This condition may result in less red blood cells being formed, and the ones that are produced are too small (microcytic)(Table1).9 Causes of iron deficiency anemia include chronic blood loss, decreased absorption of iron, or low iron intake from food.9 In iron deficiency anemia, the red blood cell count, hemoglobin, and hematocrit can be low. It should be noted that iron deficiency anemia may be considered a hypercoagulable state. This is due to increased blood viscosity through the downstream effect of microcytosis,10 along with a lowered ability of the red blood cells to deform, which can be also adversely affected by hemoglobin glycosylation in the presence of diabetes.11
Anemia of inflammation, in contrast, is mediated by the release of cytokines, tumor necrosis factor, and interleukins, which activate release of interferon 𝛽 and 𝛄 that then may lead to anemia via 3 main pathways: (1) reduction in red blood cell lifespan, (2) reduced erythropoietin response, or (3) a surplus of hepcidin production.3,4 Erythropoietin is mostly released from the kidney in response to hypoxia, which activates production of red blood cells in the bone marrow. Hepcidin is a hormone secreted by the liver that sequesters iron in the plasma regulating its availability. Anemia of inflammation may be associated with many underlying conditions, which include malignancy, kidney disease, chronic obstructive pulmonary disease, HIV, and diabetes, among others.4
Anemia can have ocular signs. Anemia can lead to retinal hypoxia, which may present as retinal edema and hemorrhages due to increased vascular permeability, along with cotton wool spots due to infarction in the retinal nerve fiber layer.12 The inner retina is more susceptible to the effects of hypoxia than the outer retina as it receives only 20% of the total volume of ocular blood flow.11
A study by Carrero et al found observable retinopathy in more than 28% of patients with anemia as defined by the World Health Organization, and the risk of anemic retinopathy may increase with the severity of the anemia.13 Anemia attributed to blood loss has a higher prevalence of retina findings than with chronic forms of anemia.13
The pathophysiology behind the retinopathy (as a consequence of anemia or other retinal hypoxic conditions) is that a low-oxygen environment in the inner retina can lead to the production of hypoxia-inducible factor 1-alpha, which induces production of vascular endothelial growth factor and nitric oxide synthase.14 The immediate effects of this chemical chain lead to retinal edema and in chronic situations may lead to angiogenesis and macular edema.7,14 Systemic vascular endothelial growth factor levels are upregulated and have been measured to be higher in patients with anemia, and in the setting of diabetes, chronic anemia can lead to presumed progression of diabetic retinopathy.7,15
Retina findings in our patient consisted of a mix of blot hemorrhages, Roth spots, and a generalized increase in overall retina thickness, including macular edema. Roth spots, or hemorrhages with a white center, are the most common type of retinal hemorrhage associated with anemic retinopathy; however, anemia can be associated with retinal hemorrhages at any level of the retina.16 Underlying anemia should also be considered in the differential diagnosis of cotton wool spots, as well as other retinal entities, including central retinal vein occlusion.10
Anemic retinopathy may be asymptomatic, but it can also present with ocular symptoms. Patients may present with vision loss, especially in cases with macular edema or optic disc edema. Our patient presented with complaints of blur at near. This may have been due to the mild hyperopic shift from macular edema. Our patient also presented with photophobia. Her anterior segment was unremarkable. She did report severe headaches associated with her episode of anemia, which may have caused her aversion to light during this time. Studies on light processing in migraine headaches have discovered that there are retina ganglion cells whose axons project to cortical and thalamic regions of the brain known as the “pain matrix.”17 These signals can generate parasympathetic and sympathetic responses from light stimulation, which may explain symptoms of photophobia and light aversion associated with migraine and concussion.17 This could possibly explain our patient’s aversion to light based on the generalized retina edema. As her anemia improved, so did her symptoms of headache and photophobia.
The first step to treating anemia should be the identification and correction of the underlying pathology, as in our patient’s case to find and correct the area of gastrointestinal bleeding.18 In mild and asymptomatic patients with iron deficiency anemia, oral iron therapy is primarily used. Ferrous sulfate is the most common iron salt used.18 Red blood cell transfusions are needed in cases of hemodynamically unstable patients or patients with severe anemia.18 In some instances, as exemplified in this case, a patient may refuse blood transfusions and other treatments must be used.19 In these cases, a combination of high-dose recombinant erythropoietin, along with vitamin supplementation, can be used to help the body make red blood cells more quickly.20 As mentioned above, erythropoietin is glycoprotein that is produced in the kidney in response to hypoxia to help increase red blood cell production and reduce red blood cell apoptosis.21 In this case, epotein alpha (procrit) was used, which is a man-made form of the naturally occurring erythropoietin protein. This is often administered along with iron, vitamin B12, and folate to help increase the patient’s hemoglobin more rapidly.20
Our patient was treated with erythropoietin 40,000 IU, intravenous iron infusions, B12 injections, and folate supplements. Although hospitalized, her workup to find the etiology of the gastrointestinal bleed included an esophagogastroduodenoscopy, colonoscopy, and computed tomography scan of the abdomen and pelvis; however, the source of the gastrointestinal bleed was not found. Prior to release from the hospital, her rectal bleeding resolved spontaneously, and the patient was no longer having bloody stools. She was prescribed ferrous sulfate 325 mg daily and folic acid 1 mg daily on release from the hospital. She was scheduled with a gastroenterologist in an outpatient clinic for follow-up.
Treatment for anemic retinopathy is directed at addressing the underlying cause of anemia.12 Our patient’s retina recovery occurred at a rapid pace consistent with her ability to rebound from her blood loss as evidenced by her improved red blood cell indices (Figure 6). Despite refusing transfusion, the resolution of the gastrointestinal bleed, along with the patient’s systemic treatment of erythropoietin, iron, folate, and B12 supplementation, elevated her hemoglobin and is attributable to her quick recovery. Her long-term retina and visual stability will depend in part on her ability to manage her diabetes and gastrointestinal integrity.
Differentials in this case included worsening diabetic retinopathy and retinal vein occlusion. In our patient’s scenario, the case history played a key role in arriving at the diagnosis. Anemic retinopathy may present with Roth spots and intraretinal hemorrhages that are larger and deeper in the retina.12 Additionally, the rate of resolution of the retina findings can also provide insight to the underlying etiology as demonstrated by our patient’s reduction of retinal edema corresponding to her improved red blood cell indices.
CONCLUSION
Anemia can be caused by acute blood loss. The patient history, laboratory data, and retina findings yielded the diagnosis of anemic retinopathy in the presence of other chronic health conditions. Our patient’s retina findings were similar to a bilateral central retinal vein occlusion and diabetic retinopathy but was distinguished by patient history and rapid recovery of her macular edema over a very short period. Most cases of anemic retinopathy are asymptomatic, though vision loss can occur in the presence of macular edema or optic disc edema. In most cases, retina findings will resolve on their own with systemic treatment of the underlying cause of the anemia. Written informed consent was obtained for this case report. No identifiable health information was included in the case report.




_initial_examination_and_(b)_8-month_follow-up_shows_resolving_hemorrh.png)





_initial_examination_and_(b)_8-month_follow-up_shows_resolving_hemorrh.png)
