INTRODUCTION
Arteritic anterior ischemic optic neuropathy is a severe optic nerve disease strongly associated with giant cell arteritis and requires prompt recognition and timely treatment to prevent irreversible vision loss (occurring in ≤15%-25% of cases)1 on the affected side and potential contralateral involvement.2,3 Understanding the epidemiology, clinical features, diagnostic algorithm, evidence-based treatment, and natural history is crucial to manage patients with this condition.
Giant cell arteritis, also referred to as temporal arteritis, is a granulomatous vasculitis that primarily affects medium- to large-sized arteries originating from the arch of the aorta, typically observed in individuals aged 50 years and older.2 It most commonly affects the aorta, branches of the ophthalmic artery, and extracranial branches of the carotid arteries.4 Less commonly, it may affect subclavian, iliac, or vertebral arteries.2 This condition frequently coexists with polymyalgia rheumatica and manifests with many different systemic symptoms, including headaches, tenderness of the scalp, jaw claudication, and systemic manifestations like weight loss, fatigue, and fever.2
Arteritic anterior ischemic optic neuropathy is characterized by sudden and often unilateral vision loss resulting from ischemic injury to the optic nerve head. The underlying pathophysiology involves inflammatory infiltration and blockage of the posterior ciliary arteries, resulting in compromised blood flow to the optic nerve.5 Early initiation of high-dose systemic corticosteroid therapy is essential in managing arteritic anterior ischemic optic neuropathy secondary to giant cell arteritis to suppress the inflammatory process and prevent further visual deterioration. Prompt referral to the emergency department for diagnostic testing and initiation of treatment and subsequent collaborative care is crucial in achieving optimal outcomes. No identifiable health information was included in this case report.
CASE REPORT
A 75-year-old White woman presented with a complaint of sudden vision loss in her left eye, which started the previous afternoon and spread to the inferior and superior fields. She reported experiencing moderate intermittent headache, including the frontal, temporal, and occipital regions, over the past month. The patient had been evaluated for her headache at urgent care a week prior and was diagnosed with an unspecified sinus problem and received a steroid nasal spray. Additionally, she reported jaw pain, scalp tenderness, temporal tenderness, weight loss of approximately 5 pounds, loss of appetite, neck and shoulder stiffness, and fatigue over the past month. There was no fever or recent history of trauma.
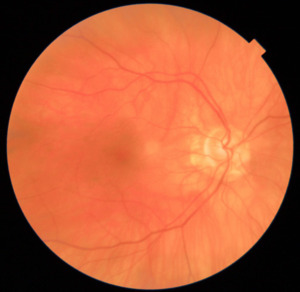
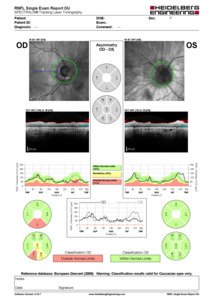
On examination, the patient’s blood pressure was 140/64 mm Hg. Her ocular history included posterior chamber intraocular lenses in both eyes, and she was being managed for glaucoma with brimonidine-timolol and latanoprost eye drops. Her medical history revealed hypertension, hyperlipidemia, and diabetes with a hemoglobin level of 7.4%. The visual acuity was right eye 20/25 right eye and left eye hand motion. Pupillary examination revealed a relative afferent pupillary defect in the left eye. Confrontation visual field testing demonstrated severe restriction in the left eye, with some remaining vision in the nasocentral and supracentral areas. Extraocular movements were full. Slit-lamp examination showed 1+ cells in the left eye. The patient experienced tenderness when the temporal artery on the left side was palpated. Intraocular pressure measurements were 13 mm Hg in both eyes. In the posterior segment, optic nerve head edema was observed in the left eye (Figure 1). Ancillary tests recommended at this point include retinal nerve fiber layer optical coherence tomography and fundus photos. The retinal nerve fiber layer optical coherence tomography confirmed optic nerve head edema.
The patient met the criteria for giant cell arteritis classification according to the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for giant cell arteritis (Table 1), including age over 50 years, morning stiffness in shoulders/neck, sudden vision loss, jaw claudication, new temporal headache, scalp tenderness, temporal artery tenderness, and palpable temporal artery. Each clinical criterion is weighed as either +3 or +2. A patient could be classified as having giant cell arteritis with a cumulative score of 6 or more points.6 The patient received a score of +13 at this time. The breakdown of points includes sudden vision loss (+3 points), morning stiffness in shoulders/neck (+2 points), jaw claudication (+2 points), a new temporal headache (+2 points), scalp tenderness (+2 points), and an abnormal temporal artery examination (+2 points). Further diagnostic confirmation at this time required an elevated erythrocyte sedimentation rate or evidence from a temporal artery biopsy. Given the urgency and potential severity of the condition, the patient was immediately referred to the emergency department. The emergency department promptly initiated a series of diagnostic blood tests, including erythrocyte sedimentation rate, C-reactive protein, and a complete blood count.
The visual prognosis for the patient at this time is expected to be poor in the left eye, with a high risk of vision loss in the other eye if immediate treatment was not initiated. The emergency department began intravenous methylprednisolone therapy for 3 days, and the patient was hospitalized for 6 days. Subsequently, the patient was switched to 60 mg oral prednisone (1 mg/kg/day), which was then gradually tapered over several months. Consultations with an endocrinologist, pathologist, ophthalmologist, internal medicine, and rheumatology specialists were ordered and completed.
The patient had a confirmed diagnosis of arteritic anterior ischemic optic neuropathy secondary to giant cell arteritis. The patient had a left temporal artery biopsy (+5 points), which showed results consistent with giant cell arteritis. Laboratory results were remarkable for elevated erythrocyte sedimentation rate and C-reactive protein (+3 points). The total score calculated based on the clinical findings, laboratory results, and biopsy findings, in accordance with the updated 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology criteria, reached a value of +21. In this case, the patient only had a temporal artery biopsy, and did not receive a temporal artery ultrasound.
Seventeen days after the initial visit, the severity of the headaches had decreased. Visual acuity was recorded as light perception in the left eye, and 20/20 in the right eye. Slit-lamp examination showed no cells. A dilated fundus examination revealed indistinct margins nasally and inferiorly in the left eye.
One month later, the patient returned to the clinic. She had tapered down her oral prednisolone dose from 60 mg to 40 mg. The patient reported difficulty with depth perception. Visual acuity was no light perception in the left eye and 20/20 in the right eye, and dilated fundus examination showed a pale disc (Figure 2).
At this point, recommendations for the patient included the use of polycarbonate lenses for safety purposes and a referral to occupational therapy to assist with navigating cooking activities. The patient was to continue regular follow-up visits with ophthalmology and rheumatology to monitor her condition and adjust treatment as needed.
DISCUSSION
Giant cell arteritis is characterized by inflammatory changes in the blood vessel wall, triggered by immune-mediated mechanisms. Although the exact cause is still unclear, there are genetic and environmental factors that may be potential contributors, such as advancing age (>50 years) and Scandinavian ancestry. The underlying mechanism of giant cell arteritis also involves an inappropriate response of the body to vascular endothelial injury. The initial endothelial injury can be from trauma or infection or be drug related or autoantigen related. 2,7,8
This endothelial injury will activate the dendritic cells that reside in the adventitia, the outermost layer of the wall of a blood vessel. These activated dendritic cells secrete cytokines, such as interleukin 6 and interleukin 18, which activate T cells to release interferon gamma. The release of interferon gamma promotes inflammation, macrophage activation, and the formation of granulomas. Activated macrophages produce matrix metalloproteinase and oxygen-free radicals, leading to endothelial damage and disruption of the internal elastic lamina. They also secrete nitric oxide within the intima, the innermost coat of a blood vessel, and fuse together to form syncytia, a multinucleated cell, resulting in the formation of “giant cells.” The macrophages also contribute to systemic inflammation by releasing interleukin 1 and interleukin 6. ^2,7, 8^
It is noteworthy that the American College of Rheumatology initially developed the widely used criteria for giant cell arteritis in 1990. These criteria have been subsequently revised in the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for giant cell arteritis. The updated criteria use a weighted algorithm that incorporates clinical, laboratory, imaging, and biopsy criteria. However, it is crucial to acknowledge that the American College of Rheumatology/European Alliance of Associations for Rheumatology advises against relying solely on the classification criteria for giant cell arteritis to establish a clinical diagnosis. Their primary purpose is to distinguish giant cell arteritis from other types of vasculitis in research settings, and they may not possess sufficient sensitivity and specificity for diagnosing individual patients. Nevertheless, these criteria can serve as a useful guide for clinicians, reminding them of the factors that are relevant to the diagnosis of giant cell arteritis. 9 When giant cell arteritis is suspected further diagnostic investigation should be initiated. This may include blood tests, and a temporal artery biopsy or temporal artery ultrasound.10
Blood work, an integral component of the diagnostic process for giant cell arteritis, involves key tests such as erythrocyte sedimentation rate, C-reactive protein, and complete blood count. Elevated erythrocyte sedimentation rate and C-reactive protein levels indicate systemic inflammation, often observed in giant cell arteritis. The 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria set specific threshold values of more than 50 mm/h for erythrocyte sedimentation rate and more than 10 mg/L for C-reactive protein. These values are based on evidence demonstrating their high sensitivity and specificity for detecting giant cell arteritis–related inflammation.
The 2022 guidelines do not explicitly use sex and age to calculate values because several reasons. Firstly, using specific cutoff values simplifies and streamlines the diagnostic process, making it more universally applicable and less reliant on individual patient characteristics. Secondly, research has indicated that absolute thresholds for erythrocyte sedimentation rate (>50 mm/h) and C-reactive protein (>10 mg/L) may be more reliable and practical for diagnosing giant cell arteritis across different populations, irrespective of age or sex variations in baseline values. Lastly, the age component is not factored in because it is already a prerequisite for considering a giant cell arteritis diagnosis according to the 2022 criteria. ^6, 11^
Platelet levels, also commonly monitored, contribute to assessing inflammation severity and potential ischemic risk. Platelet counts exceeding 300 × 10^9 per liter are often considered elevated. However, interpretation requires consideration within the broader clinical context, as elevated platelet levels are not specific to giant cell arteritis.12
Temporal artery biopsy and temporal artery ultrasound are commonly used diagnostic procedures for giant cell arteritis. Temporal artery biopsy involves the surgical extraction and microscopic examination of a temporal artery segment to identify inflammatory multinucleated giant cells affecting the arterial wall. These histological changes, including intimal hyperplasia, disruption of the internal elastic lamina, and inflammatory infiltrates predominantly composed of lymphocytes, macrophages, and multinucleated giant cells, are diagnostic features of giant cell arteritis. Importantly, the specificity of temporal artery biopsy is generally considered high, often exceeding 90%, indicating that a positive biopsy result is highly indicative of giant cell arteritis.13
Temporal artery ultrasound offers several advantages over temporal artery biopsy in the diagnostic evaluation of giant cell arteritis. Temporal artery ultrasound is noninvasive, making it suitable for use in various clinical settings, including the emergency department, where prompt diagnosis is crucial to prevent sight-threatening complications. It is widely available, cost-effective, and relatively easy to perform, enhancing its utility as a frontline investigation for suspected giant cell arteritis cases. Importantly, temporal artery ultrasound demonstrates high sensitivity and specificity for diagnosing giant cell arteritis, with reported values of 89% and 94%, respectively. The inclusion of temporal artery ultrasound findings, such as the halo sign, in the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology criteria underscores its importance as a diagnostic tool. The halo sign, characterized by hypoechoic vessel wall thickening of the intima-media complex, reflects the granulomatous inflammation typical of giant cell arteritis. Despite these advantages, temporal artery ultrasound may lack the definitive histological confirmation provided by temporal artery biopsy, leading to potential false-positive results and the need for further clinical correlation in certain cases.14,15
Variability is observed in the dilation of the central veins between the initial and subsequent visits. Although this variation might raise the possibility of a central retinal artery occlusion, it is essential to contextualize these findings within the broader clinical picture. Venous dilation is more commonly associated with arteritic anterior ischemic optic neuropathy rather than central retinal artery occlusion.16 In arteritic anterior ischemic optic neuropathy, impaired blood flow to the optic nerve head leads to optic disc edema, which can cause compression of the adjacent retinal veins, resulting in venous dilation. In contrast, central retinal artery occlusion primarily affects the arterial circulation of the retina and does not typically result in venous dilation.
The typical appearance of arteritic anterior ischemic optic neuropathy involves optic disc edema with a pale “chalky white” appearance, yet our patient exhibits optic disc edema without displaying this chalky white appearance. Conversely, the typical appearance of central retinal artery occlusion involves the classic “cherry-red spot” appearance on fundoscopy due to retinal ischemia. In the case of this patient, the presence of optic disc edema without a cherry-red spot is more indicative of arteritic anterior ischemic optic neuropathy. The absence of a chalky white appearance may indicate that the edema is not severe enough to cause visible pallor of the optic nerve head. Alternatively, the lack of a chalky white appearance could suggest that the edema is still in the early stages, before significant axonal loss and optic nerve head pallor have occurred. In this case, the retinal nerve fiber layer optical coherence tomography from the initial visit showing a thickness range of 102 to 145 microns in the temporal quadrant suggests some degree of retinal nerve fiber layer thickening, possibly indicative of early optic nerve edema. The subsequent visit, 17 days later, demonstrates further thickening in the temporal quadrant (121-169 microns) and a progression from 71 to 119 microns in the supranasal quadrant. These findings suggest ongoing or worsening optic nerve edema. It is essential to note that optic disc appearance can vary widely among individuals, and the absence of a chalky white appearance does not rule out the possibility of optic nerve head.
During a 4-year period from January 2018 to January 2022, a retrospective cross-sectional study aimed to assess the diagnostic performance of the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for giant cell arteritis in routine clinical practice. Although originally designed for patient classification in research settings, it is important to evaluate the criteria’s effectiveness in routine care and compare it with the classic 1990 American College of Rheumatology giant cell arteritis classification criteria. The study focused on patients referred to fast-track clinics at 2 academic centers, with the purpose of screening for potential giant cell arteritis cases using temporal artery ultrasound. This research aimed to provide valuable insights into the utility of the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology giant cell arteritis classification criteria for diagnosing patients with suspected giant cell arteritis in routine clinical practice. 17
This retrospective study represents a significant milestone as it provides the first external validation of the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology giant cell arteritis classification criteria in routine clinical practice. The aim of the study was to assess the effectiveness of these criteria in diagnosing patients with suspected giant cell arteritis. The results demonstrate that the new criteria perform well in supporting the clinical diagnosis of giant cell arteritis and show an improvement in diagnostic accuracy compared with the older 1990 American College of Rheumatology giant cell arteritis classification criteria.
Once suspicion of arteritic anterior ischemic optic neuropathy secondary to giant cell arteritis is confirmed timely corticosteroid treatment should be initiated. Prompt corticosteroid treatment in patients with arteritic anterior ischemic optic neuropathy secondary to giant cell arteritis improves visual prognosis by reducing arterial inflammation, alleviating ischemic damage to the optic nerve, and preventing further vision loss.4 High-dose intravenous corticosteroids are typically administered initially to rapidly suppress the inflammatory response. After the acute phase, the dosage is gradually tapered to a lower maintenance dose to prevent relapses while minimizing the potential side effects of long-term corticosteroid use.
The decision to initiate treatment using either oral or intravenous steroids is dependent on the presence of visual symptoms/loss or critical cranial ischemia. Patients manifesting signs of impending vision loss or who have already experienced vision loss will be administered intravenous methylprednisolone at a dosage of 500 to 1000 mg/day for 3 to 5 days, followed by a transition to oral prednisone of 1 mg/kg up to 80 mg daily. The oral prednisone dosage is gradually tapered with a reduction of around 10 mg per month until a daily dose of 40 mg is reached. The tapering rate is adjusted to 5 mg per month until a daily dose of 10 mg is achieved. From there, the dosage can be further reduced by 1 to 2.5 mg per month until a daily maintenance dose is reached or until the corticosteroid is discontinued.18,19
The use of corticosteroids is a mainstay of treatment for giant cell arteritis. However, tocilizumab, an interleukin 6 inhibitor, has been approved by the US Food and Drug Administration and has shown promising results for the treatment of giant cell arteritis. Tocilizumab is a monoclonal antibody that targets the interleukin 6 receptor. This inhibition of the signaling pathway of interleukin 6 suppresses the downstream inflammatory response. Intravenous weekly, or biweekly, treatment with tocilizumab has demonstrated efficacy in achieving remission, reducing relapse rates, and sparing corticosteroid use.20,21 The 2021 American College of Rheumatology guidelines recommend considering using tocilizumab as a monotherapy or in combination with corticosteroids. The decision to include tocilizumab in initial treatment depends on the patient’s clinical status, underlying conditions, and physician’s judgement.19,20,21
It is crucial to recognize that arteritic anterior ischemic optic neuropathy secondary to giant cell arteritis is associated with a high risk of bilateral involvement. The affected eye may already be compromised, and the fellow eye is at risk of developing arteritic anterior ischemic optic neuropathy in the future.22 Therefore, close monitoring and ongoing management are essential to detect any changes or signs of involvement in the fellow eye.
Long-term visual prognosis depends on several factors, including the severity of the initial vision loss, the response to treatment, and the presence of systemic complications related to giant cell arteritis.22 Systemic complications associated with giant cell arteritis include polymyalgia rheumatic, large-vessel vasculitis, and other constitutional symptoms. Approximately 60% of patients with giant cell arteritis may also develop polymyalgia rheumatic, an inflammatory condition characterized by muscle pain and stiffness around the shoulders and hips. About 20% of patients with giant cell arteritis may develop large-vessel involvement, which may lead to aortic aneurysms, dissection, arterial bruit, or claudication. Less than 15% of patients with giant cell arteritis may have ischemic manifestations of the central nervous system, such as strokes or transient ischemic attacks.23
The outcomes vary; although some patients may experience stable vision with proper management, others may continue to have gradual visual decline over time. Regular follow-up visits and adherence to treatment regimens are crucial to optimize visual outcomes and minimize the risk of recurrence or progression of the disease. It is important to note that each patient’s situation is unique, and individual prognosis may vary. Therefore, comprehensive evaluation, timely intervention, and ongoing care are essential to ensure the best possible visual prognosis for individuals with arteritic anterior ischemic optic neuropathy secondary to giant cell arteritis.
CONCLUSION
In clinical practice, although the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology giant cell arteritis classification criteria are not intended for diagnostic purposes, they can serve as a valuable aid to assist in the diagnosis of giant cell arteritis. Incorporating these criteria into the diagnostic process can enhance the accuracy and efficiency of giant cell arteritis diagnosis for health care professionals. This, in turn, enables timely initiation of appropriate management strategies, leading to improved patient outcomes. Once giant cell arteritis is considered as a potential cause of vision loss, the next crucial step is emergent referral for further investigation. This should include blood tests and diagnostic procedures, such as temporal artery biopsy or temporal artery ultrasound, to confirm the diagnosis accurately.10
Although this case validates the effectiveness of the new classification criteria, further research is still necessary. It is important to assess the diagnostic accuracy of the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology giant cell arteritis classification criteria in diverse populations with giant cell arteritis. Conducting additional studies will help determine the generalizability and reliability of these criteria across different patient cohorts. Overall, the validation of the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology giant cell arteritis classification criteria in routine clinical practice underscores their value in supporting the diagnosis of giant cell arteritis and highlights their potential to enhance patient care.



_illustrates_improved_.png)


_illustrates_improved_.png)

