INTRODUCTION
The cornea is innervated by the ophthalmic division of the trigeminal nerve.1 This innervation is responsible for sensation, epithelial wound healing, tear production, blink reflex, and trophic functions.1 Neurotrophic keratitis describes disruption of this innervation and reduced corneal sensation, which can be categorized by the Mackie classification system as mild punctate keratopathy (stage 1), a persistent corneal epithelial defect (stage 2), corneal ulceration (stage 3), or even corneal perforation.2,3 Trigeminal nerve impairment may be acquired or congenital. Complete loss of trigeminal innervation from birth may be referred to as congenital trigeminal anesthesia, which is often associated with severe vision loss.2,4 Often it manifests as conjunctival redness, nonhealing or recurrent corneal epithelial defects, and corneal scarring.5 The most common causes of congenital trigeminal anesthesia include posterior fossa tumors, cerebellar hypoplasia, head trauma, Goldenhar syndrome, familial dysautonomia, and that of idiopathic origin.5
We present a case of a neurotrophic ulcer in a child with a congenital hypoplastic trigeminal nerve and concurrent septo-optic dysplasia with pituitary and gray matter abnormalities. This case report details treatment of this neurotrophic ulcer using the combination of amniotic membrane transplantation, tarsorrhaphy, allogenic serum eye drops, recombinant human nerve growth factor, and corneal neurotization surgery. No identifiable health information was included in this case report.
CASE REPORT
A boy aged 3 years with septo-optic dysplasia and developmental delay presented for a bilateral myringotomy and pressure-equalizing tube placement surgery, along with an ophthalmology consultation under anesthesia, for a nonhealing corneal ulcer of the right eye despite treatment with topical antibiotics and corticosteroids during the prior several months by an outside ophthalmologist. Examination revealed 3+ injection of the conjunctiva, a 5 mm by 5 mm corneal ulcer (Figure 1) with an epithelial defect and central corneal opacification. He was treated with a cryopreserved amniotic membrane (Prokera: Miami, Florida: BioTissue) and a temporary suture tarsorrhaphy. The ulcer was suspected to be neurotrophic given the chronicity and lack of pain.
On day 31, the tarsorrhaphy and the amniotic membrane were removed. Examination demonstrated no improvement in the corneal defect size, no infiltrate, and 5% to 10% corneal thinning (Figure 2). Visual acuities were difficult to assess given age and developmental delay and were reported as “fix and follow” in each eye individually at each visit. A bandage contact lens was placed on the right eye and the patient was started on moxifloxacin 4 times per day, and erythromycin ophthalmic ointment as needed for lubrication. The child was also prescribed recombinant human nerve growth factor (Oxervate, 0.002% cenegermin-bkbj ophthalmic solution: Milano, Italy: Dompe farmaceutici) to use 6 times per day in the right eye on medication arrival. Extraocular muscle movements and confrontation visual fields were normal. The left pupil was round and responsive to light, whereas the right pupil was unable to be assessed because of corneal scarring.
Five days later, the corneal defect of the right eye was slightly improved. The recombinant human nerve growth factor had not arrived. Allogenic serum eye drops, from parental serum, were prescribed to be used 4 times per day, and the bandage contact lens was replaced as it had fallen out the day after being inserted. The patient was to continue using moxifloxacin 4 times per day and erythromycin at nap and at night and to patch the right eye when able. Preservative-free artificial tears were also recommended to be used every 1 or 2 hours in the right eye.
On day 50, the bandage contact lens was in place and the patient had a smaller epithelial defect measuring 4mmx3mm. The patient had not started the allogenic serum eye drops nor the recombinant human nerve growth factor eyedrops. At this visit, the allogenic serum eye drops had just arrived, and the parents were instructed to begin instilling them 4 times per day. The bandage contact lens was also replaced.
On day 59, the corneal epithelial defect remained stable with no infiltrate. However, inferior neovascularization of the cornea was forming. The recombinant human nerve growth factor had arrived, and parents were instructed to instill 6 times per day along with allogenic serum eye drops 4 times per day and moxifloxacin 4 times per day. The bandage contact lens was removed.
On day 66, the corneal epithelial defect was completely resolved and the inferior neovascularization was receding. The plan was to continue recombinant human nerve growth factor 6 times per day for the next 7 weeks and continue allogenic serum eye drops 4 times per day indefinitely. The moxifloxacin was discontinued at this visit.
At day 136, the patient had been off recombinant human nerve growth factor for roughly 2 weeks. The cornea was still epithelialized, and a central corneal opacity remained (Figure 3). The plan was to continue allogenic serum eye drops 4 times per day indefinitely.
Unfortunately, in the next 2 years the patient experienced repeated self-inflicted corneal abrasions, which required daily bandage contact lens wear and patching of the right eye when possible. Because of these repeated injuries and risk of further corneal complications, it was decided that the best option was corneal neurotization surgery of the right trigeminal nerve. On day 954, he underwent corneal neurotization using the right sural nerve and contralateral superior orbital nerve. At his most recent follow-up on day 1401, his right cornea was free of epitheliopathy with a treatment consisting only of preservative-free artificial tears as needed. There was also an improvement of the corneal scarring. Clinicians and guardians of the child had no concerns regarding adherence throughout the treatment course.
DISCUSSION
Congenital neurotrophic keratitis or congenital trigeminal anesthesia generally presents during infancy or within the first few years of life as eye redness, nonhealing corneal epitheliopathy or defects, and corneal scarring. In cases of discordance in which a child is experiencing minimal symptoms with significant corneal findings, congenital trigeminal anesthesia should be included in the differential diagnosis list. This is especially true if there is any accompanying brain malformation, cranial nerve palsies, facial dysmorphism, developmental delay, or nonhealing skin lesions along the trigeminal nerve distribution. Congenital trigeminal anesthesia can be subdivided into 3 types based on trigeminal nerve embryology.6
Rosenberg Classification of Congenital Trigeminal Anesthesia6
-
Type I: isolated
-
Type II: ectodermal or mesodermal maldevelopment
-
Type III: focal brainstem pathology potentially from a vascular prenatal event without evidence of systemic developmental abnormalities
Although the sensory portion of the trigeminal nerve arises from the neural crest, the motor portion develops from the neural tube.7 The neural crest is responsible for mesodermal and some ectodermal development, which explains why congenital trigeminal anesthesia may be associated with skull, spine, or ear malformations.8 In this classification system, type I is considered an isolated event and confined to the cornea only, type II is related to mesodermal and ectodermal embryonic development, and type III is related to focal brainstem malformations secondary to prenatal injury, likely vascular in nature. In type I, the key classification is the absence of other neurological or mesoectodermal congenital abnormalities. This type typically only involves the ophthalmic division of the trigeminal nerve and etiology is thought to be due to primary hypoplasia of the trigeminal nuclei/hindbrain. Bilateral cases are more common than unilateral. Type II is associated with mesodermal and/or ectodermal developmental disorders such as Goldenhar syndrome or oculoauriculovertebral spectrum, Moebius syndrome, VACTERL association (vertebral, anal, cardiovascular, tracheoesophageal, renal, and limb defects), MURCS association (Müllerian duct aplasia–renal agenesis–cervicothoracic somite dysplasia), Riley-Day syndrome (familial dysautonomia), and congenital insensitivity to pain. This type can be unilateral, more commonly seen in patients with Goldenhar syndrome, or bilateral, more common in patients with non-Goldenhar syndromes. In type III, there is a prenatal injury, likely vascular in nature, that causes focal brainstem abnormalities with no evidence of any systemic developmental abnormalities.4 An example of type III congenital trigeminal anesthesia would be a patient presenting with unilateral corneal anesthesia with ipsilateral sixth and seventh nerve palsies without systemic abnormalities caused from a prenatal ischemic insult.
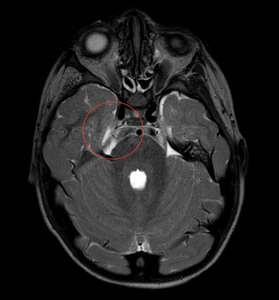
Despite no diagnosed genetic syndrome, we believe our patient has type II congenital trigeminal anesthesia given his significant medical history of septo-optic dysplasia with associated growth hormone deficiency and central hypothyroidism, global developmental delays with hypotonia, autism spectrum disorder, and sensorineural hearing loss. Magnetic resonance imaging of the brain and orbits performed when the child was aged 1 month revealed posterior pituitary ectopia, subependymal gray matter heterotopia, and left occipital subcortical gray matter heterotopia with overlying polymicrogyria, multiple cranial nerve abnormalities including bilateral optic nerve hypoplasia, a hypoplastic right trigeminal nerve, and small cochlear nerves (Figure 4). Repeat neuroimaging was performed at 4 years and 2 months of age (Figure 5) demonstrating stability of the septo-optic dysplasia and hypoplastic right trigeminal nerve.
Unfortunately, at our initial examination, our patient already had advanced corneal scarring and a neurotrophic ulcer with corneal thinning consistent with stage 3 neurotrophic keratitis. Early identification and treatment of congenital neurotrophic keratitis is essential as this may lead to fewer complications and better visual outcomes. However, even with early intervention, permanent vision loss is still common. Microbial keratitis is a concern in pediatric cases of corneal anesthesia, with approximately 45% developing an infection and 35% having an infection at presentation.5 Corneal scarring occurs in 73% of children with corneal anesthesia.5 In a 2-center retrospective cohort by Lambley with 33 eyes from 26 children with corneal anesthesia, only 15% had a final visual acuity of 20/40 or better, 30% worse than 20/200, and the rest between 20/40 and 20/200.5 Visual prognosis was worse in patients with a concomitant facial nerve palsy.5 We were unable to measure quantitative visual acuity in our patient because of age and developmental delay.
Treatment options for neurotrophic keratitis or congenital trigeminal anesthesia include lubricating eye drops, bandage contact lens wear, amniotic membrane transplantation, allogenic serum eye drops, recombinant human nerve growth factor, topical insulin, substance P, insulin-like growth factor, tarsorrhaphy, scleral contact lenses, and corneal neurotization surgery.9 The aggressiveness of the treatment is primarily dependent on the stage of the neurotrophic keratitis. In our patient, there was no improvement in corneal epithelization with amniotic membrane transplantation combined with tarsorrhaphy and the corneal epithelium only marginally healed using allogenic serum eye drops despite the epitheliotrophic growth factors present in such drops. Although tarsorrhaphy, bandage contact lens wear, and topical antibiotics prevented microbial keratitis, this reduced corneal exposure also did not resolve the neurotrophic ulcer. Although topical insulin has shown promising effects in the treatment of neurotrophic keratitis, it was not considered in this case as there was minimal research supporting its use at the time and because of its limited availability.10 The recalcitrant neurotrophic ulcer healed in 1 week or less with recombinant human nerve growth factor presumably because of the higher concentration of nerve growth factor present in recombinant human nerve growth factor as compared with allogenic serum eye drops. Early evidence demonstrates the effectiveness of recombinant human nerve growth factor in the treatment of pediatric neurotrophic keratitis.11,12 Corneal neurotization is a surgery that uses a local nerve or graft to reinnervate an anesthetic or hypoesthesic cornea.13 When the sural (afferent) nerve is used, this is considered an indirect interposition technique rather than a direct transfer procedure (contralateral vs ipsilateral). Despite the problem originating upstream in the trigeminal ganglion, reinnervation to the downstream corneal nerves is possible via retrograde neuronal effects from the rerouted donor nerve.14 This new innervation of the corneal nerves delivers protection and viability to the ocular surface. Preliminary evidence demonstrates that neurotization surgery yields clinically significant improvements in corneal sensation, visual acuity, and ocular surface integrity.13 because of repeated corneal injury, our patient eventually underwent corneal neurotization surgery, which was successful. Eyes with corneal anesthesia are particularly susceptible to traumatic epithelial injury because of reduced sensation as well as reduced epitheliotrophic factors secreted by the corneal nerves.10 Such epithelial damage predisposes these corneas to infection, ulceration, scarring, and perforation, which increase the risk of deprivation and refractive amblyopia in children.
Patients with congenital trigeminal anesthesia should be screened for brain malformations, mesodermal and ectodermal abnormalities, and skeletal deformities. Our patient had a brain magnetic resonance imaging prior to our initial examination and was diagnosed with septo-optic dysplasia and a hypoplastic right trigeminal nerve among other brain malformations. Parents or guardians should be educated on the risk of injury to the face and eye due to reduced or absent trigeminal sensation. Prior to corneal neurotization surgery, we attempted to mitigate this risk using daily bandage contact lens wear and patching the eye but were ultimately unsuccessful in preventing corneal injury.
CONCLUSION
Although pediatric cases of neurotrophic keratitis are relatively rare, they tend to be severe and recalcitrant. This case used amniotic membrane transplantation, topical antibiotics, bandage contact lenses, allogenic serum eye drops, preservative-free artificial tears, recombinant human nerve growth factor, and finally, corneal neurotization surgery. This case adds to the growing body of evidence for the effectiveness of recombinant human nerve growth factor in healing pediatric neurotrophic ulcers and corneal neurotization surgery for restoration of trigeminal nerve function. No identifiable health information was included in this case report.
TAKE HOME POINTS
-
This is a challenging case of a 3-year-old patient with a refractory neurotrophic corneal ulcer secondary to a rare congenital trigeminal anesthesia.
-
The use of recombinant human nerve growth factor resulted in the resolution of the neurotrophic ulcer in just one week, highlighting the effectiveness of this treatment approach.
-
Corneal neurotization surgery was successful in restoring corneal nerve function, suggesting its potential as a long-term treatment option for congenital neurotrophic keratitis.
ACKNOWLEDGEMENTS
The authors thank Austin Larson, MD, for his genetic expertise and insight into the embryonic development pertaining to this case.